The following points highlight the two main methods of transferring transgene into the target plants. The methods are: 1. Vector Mediated Gene Transfer 2. Vector less or Direct Gene Transfer.
Method # I.
Vector Mediated Gene Transfer:
In vector mediated gene transfer the transgene is transferred to the target plant by the help of a DNA vector. The vector could be either the plasmids obtained from Agrobacterium tumefaciens or the DNA of plant viruses.
Agrobacterium Mediated Gene Transfer:
A. tumefaciens (AT) is a soil microorganism that causes crown gall disease in many species of dicotyledonous plants. The Ti plasmid isolated from AT is widely used as vector in the generation of transgenic plant.
Basis of Tumour Formation:
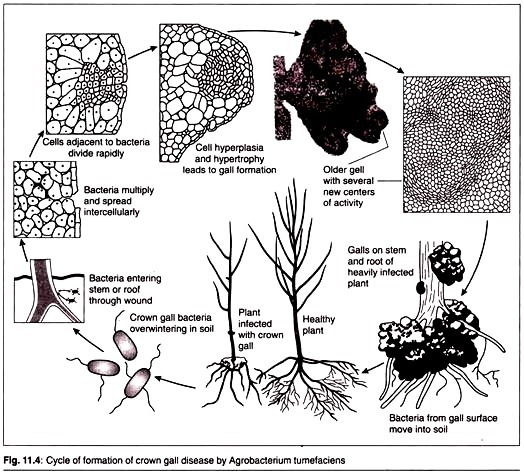
Crown gall occurs when a wound on the stem allows A. tumefaciens bacteria to invade the plant. After infection the bacteria cause a cancerous proliferation of the stem tissue in the region of the crown.
During the end of 1970s three microbiologists Mary-Dell Chilton , Eugene W. Nester, and Milton P. Gordon proved that it was DNA from a plasmid in AT that was transferred and integrated into the plant DNA to cause crown gall tumours. The plasmid that was responsible for the tumour was named as Ti (tumour inducing) plasmid. The process of gall formation is shown in diagram below.
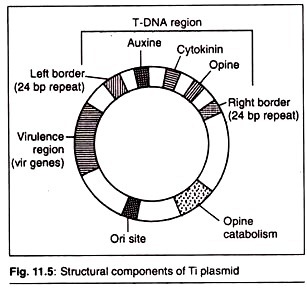
Structure of Ti Plasmid:
A Ti plasmid has five important regions:
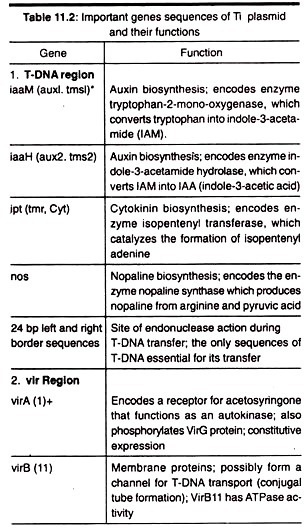
1. T-DNA Region:
This region has the genes for the biosynthesis of auxin (aux), cytokinin (cyt) and opine (ocs), a type of amino acid derivative. This is flanked by left and right borders. The aux, cyt and ocs as a whole considered as oncogenes as their products are responsible for the formation of tumour. The opine produced by the Ti plasmids can be any one of the four types, they are octopine, nopaline, succinamopine and leucinopine.
2. T-DNA Borders:
The T-DNA is flanked by left and right borders, each of 24 base pairs nucleotides. Now it is known that the right border is more critical for T-DNA transfer and tumour formation.
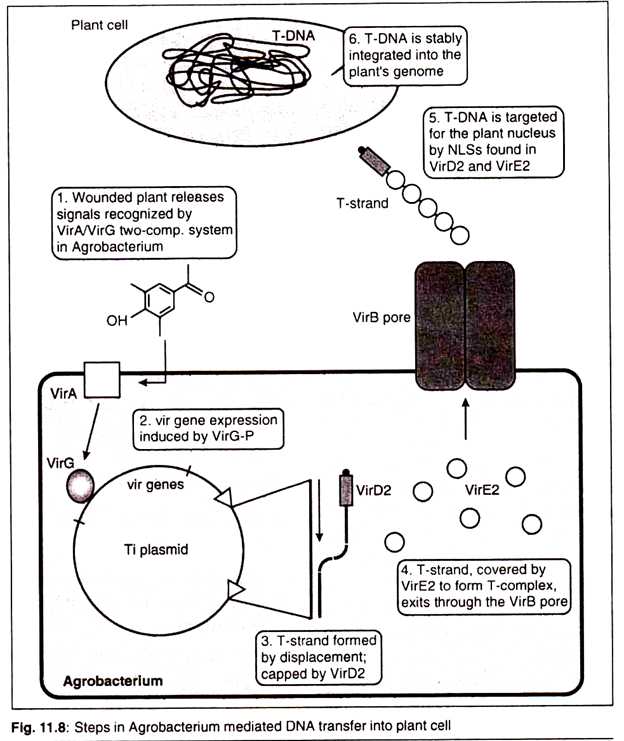
3. Virulence Region:
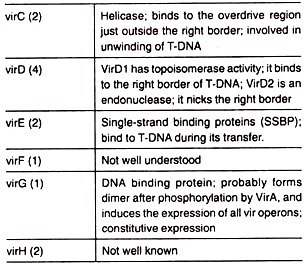
This zone has certain genes known as vir genes which are responsible for the transfer of T-DNA into the host cell (plant cell). At least nine vir gene operons have been identified.
4. Opine Catabolism Region:
This region codes for proteins involved in the uptake and metabolism of opines.
5. Ori Region:
Origin of replication and necessary for the replication of the Ti plasmid.
Using the Ti Plasmid to Introduce New Genes into a Plant Cell:
While using the Ti plasmid as a vector we have to introduce our transgene in the T-DNA region and then the bacterium could do the hard work of integrating them into the plant chromosomal DNA. The main problem with this operation is that a unique restriction site is an impossibility with a plasmid 200 kb in size.
Following two strategies have been developed for inserting the transgene into the Ti plasmid:
1. The Binary Vector Strategy
This strategy is fundamentally based on the fact that the T-DNA does not need to be physically attached to the rest of the Ti plasmid. A two-plasmid system has the T-DNA on a relatively small plasmid and the rest of the part on another one. The T-DNA plasmid is small enough to have a unique restriction site and to be manipulated using standard techniques.
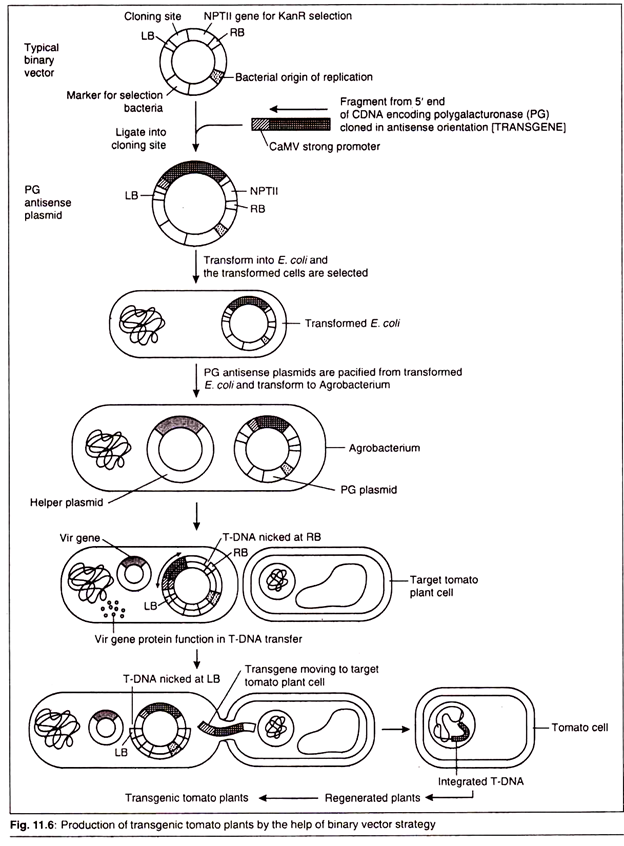
The binary system has been widely used for the production of tomatoes that ripened more slowly and so would not bruise during transport to supermarkets and will also have a long shelf life. From the research it has been found that the quick ripening of tomatoes has a direct correlation with an increased level of an enzyme, polygalacturonase (PG), in its cell’s cytoplasm.
So if we can control the level of PG in the cytoplasm of tomato cells then we can delay their ripening.
This is achieved by developing transgenic tomato plants having a transgene that can code an antisense RNA for the mRNA of PG which are naturally produced in their cells. This can be done by the help of binary system strategy where we take two types of plasmids – the T-DNA plasmid from Agrobacterium having left border (LB) and right border (RB), neomycin phosphotransferase II (NPTII) as selectable markers and a helper plasmid, which is a modified Ti plasmid that is missing its T-DNA but still contains vir genes.
The process of development of transgenic tomato plants can be carried out by observing following steps:
Step 1:
Introduction of the transgene, having a stronger promoter from CaMV and ORF for PG, in the cloning site of a binary vector. This results in the production of PG antisense plasmid.
Step 2:
The PG antisense plasmid is then transformed to E. coli cells for its cloning. The transformed E. coli cells can be selected on the basis of antibiotic selection method (as the antibiotic is encoded by the marker for selection in bacteria present on PG antisense plasmid).
Step 3:
The PG antisense plasmid is then isolated and purified from E. coli cells and then transformed into Agrobacterium.
Step 4:
The transformed Agrobacterium has two types of plasmids – the PG antisense plasmid and the modified Ti plasmid having only vir genes. By the help of vir gene products the T-DNA region of the PG antisense plasmid gets transferred to the target tomato plant cell.
Step 5:
The transformed plant cell is selected on the basis of kanamycin antibiotic resistant selection.
Step 6:
The transformed cells are subjected to plant tissue culture technique and the transgenic tomato plant is regenerated.
Advantages:
Compared with co-integrated vectors, binary vectors present some advantages:
a. No recombination process takes place between the molecules involved.
b. Instead of a very large, recombinant, disarmed Ti plasmid, small vectors are used, which increases transfer efficiency from E. coli to Agrobacterium.
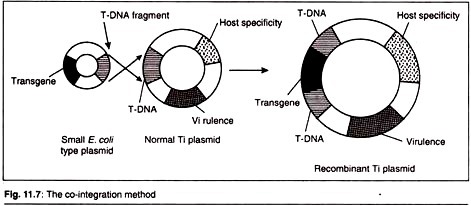
2. The Co-Integration Strategy:
This procedure uses an entirely new plasmid just like E. coli vector which carries a small portion of the T-DNA. The process of genetic recombination can integrate the E. coli plasmid into the T-DNA region. The gene to be cloned is therefore inserted into a unique restriction site on the small E. coli plasmid which gets introduced into A. tumefaciens cells carrying a Ti plasmid.
Advantages:
a. The target genes can be easily cloned.
b. The plasmid is relatively small with a number of restriction sites.
c. Intermediate plasmid is conveniently cloned in E. coli and transferred to Agrobacterium.
Production of Transformed Plants with the Ti Plasmid:
When A. tumefaciens bacteria containing an engineered Ti plasmid are introduced into a plant by infection of a wound in the stem then only the cells in the resulting crown gall will possess the cloned gene. In the generation of the transgenic plant we need every cell of the plant to have the transgene.
To achieve this there are several solutions, the most widely method employed for this, however, is very simple. In this technique we infect a culture of plant cells or protoplasts in liquid medium.
This generates a mixed population of plant cells some of which are transformed and some other are non-transformed in nature. To identify the transformed plant cells, all the cells in the culture are grown on selective medium.
The transformed cells which are selected are subjected to plant tissue culture protocol in order to generate a whole plant. It should be remembered that regeneration of a transformed plant can occur only if the Ti vector has been “disarmed” so that the transformed cells do not display cancerous properties. Disarming is possible because the cancer genes, all of which lie in the T-DNA, are not needed for the infection process.
The only parts of the T-DNA that are involved in infection are two 25 bp repeat sequences found at the left and right borders of the region integrated into the plant DNA. Any DNA placed between these two repeat sequences will be treated as “T-DNA” and transferred to the plant.
Hence, we can remove all the cancer genes from the normal T-DNA and replace them with an entirely new set of genes without hampering the infection process.
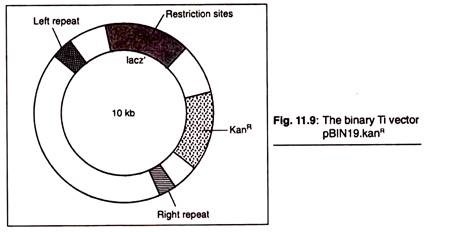
Nowadays a number of disarmed Ti cloning vectors are available for direct use. Example of such vectors is the binary vector pBIN19. The left and right T-DNA borders present in this vector flank a copy of the lacZe gene, containing a number of cloning sites, and a kanamycin resistance gene that functions after integration of the vector sequences into the plant chromosome.
Advantages of Agrobacterium mediated transformation:
a. This method is a natural method of gene transfer so needs less of human input for its success.
b. AT has a tendency to infect any ex-plant cells.
c. It is successful in transferring even a larger fragment of transgene.
d. The gene is transferred in a stable manner.
e. The regeneration of the transferred plant is effective.
Disadvantages of Agrobacterium mediated transformation:
a. AT has a narrow range of host cells.
b. Some tissues in the plant are not transformed by the AT. e.g., cells present in the deeper layers of embryo.
The Ri Plasmid:
Ri plasmid isolated from Agrobacterium rhizogenes is also used as vector for the generation of transgenic plants. Ri and Ti plasmids are very similar. The important difference being that transfer of the T-DNA from an Ri plasmid to a plant results not in a crown gall but in hairy root disease. This disease is characterized by a massive proliferation of a highly branched root system.
Plant Viruses as Cloning Vectors:
Viruses are the most common pathogens of the plants. Their infection mechanism has developed excellent means of delivery of their genome into the plant cells. If we can use this delivery mechanisms at the safer manner, not developing any plant disease, then viruses would be much more convenient to use than other types of vector, because with many viruses transformation can be achieved simply by rubbing the virus DNA onto the surface of a leaf. Two classes of DNA virus are known to infect higher plants; the caulimoviruses and Gemini viruses, are helpful in the generation of transgenic plants.
(a) Caulimovirus Vectors:
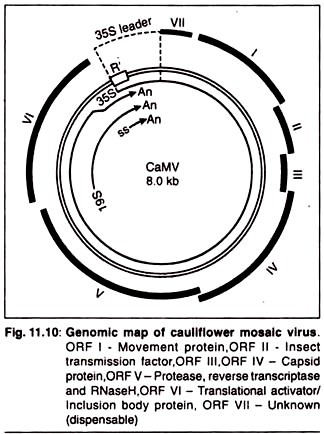
The caulimoviruses group consists of 6-19 viruses, each of which has a relatively limited host range. The best known member, cauliflower mosaic virus (CaMV), infects many members of Cruciferae (cabbage, cauliflower, turnips, Brussels sprouts, rapeseed, Arabidopsis, etc.) and Datura stramonium. This is one of the first virus that had been used as a vector for the generation of transgenic plants.
There are two problems associated with caulimovirus vectors:
Problem 1: Afford as Very Smaller Size of Foreign Gene (Transgene):
Even after deletion of non-essential sections of the virus genome the capacity for carrying transgene is very limited. This problem can be solved by using a helper phage. In this strategy we take the cloning vector as cauliflower mosaic virus (CaMV) genome, that lacks several of the essential genes which are necessary for infection and thus can uptake a large part of the transgene, along with a normal CaMV genome (helper phage genome) that has all the genes necessary for the initiation of infection in plants.
Problem 2: Has Extremely Narrow Host Range of Hosts:
Due to this the cloning experiment is restricted to just a few plants such as turnips, cabbages, and cauliflowers.
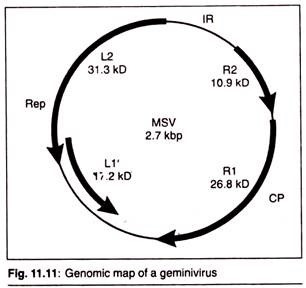
(b) Gemini virus Vectors:
Gemini viruses are particularly interesting because their natural hosts include plants such as maize and wheat which forms the major staple food of the people throughout the world.
Both curly top virus (CTV) and maize streak disease virus (MSV) are Gemini viruses having single stranded RNA as their genome. Gemini viruses have a much wider host range than CaMV. One advantage this group of viruses does have is that they contain DNA which, although single stranded, appears to replicate via a double- stranded intermediate, which would make in vivo manipulation in bacterial plasmids more convenient.
The virus group is known to infect a wide range of crop plants, including monocots and dicots. Attempts are underway to develop wheat dwarf Gemini viruses as vectors. One disadvantage with this virus is that during the infection cycle its genome undergo rearrangements and deletions, which would scramble up any additional DNA that has been inserted at the aim of the experiment will be never achieved.
Method # II.
Vector less or Direct Gene Transfer:
The followings are the different types of direct gene transfer techniques that could be used for the production of a transgenic plant:
(a) Electroporation:
This involves a pulse of high voltage applied to target plant cell to make transient (temporary) pores in the plasma membrane which facilitates the uptake of foreign DNA. The cells are placed in a solution containing DNA and subjected to electrical shocks to cause holes in the membranes. The foreign DNA fragments enter through the holes into the cytoplasm and then to nucleus.
(b) Microinjection:
In this technique the DNA is directly injected into plant protoplasts or cells (specifically into the nucleus or cytoplasm) using fine tipped (0.5 – 1.0 micrometer diameter) glass needle or micro-pipette.
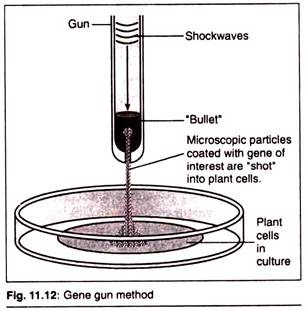
(c) Particle Gun or Particle Bombardment:
In this method, the foreign DNA containing the genes to be transferred is coated onto the surface of minute gold or tungsten particles (1-3 micrometers) and bombarded onto the target tissue or cells using a particle gun (also called as gene gun/shot gun/micro projectile gun). The micro projectile bombardment method was initially named as biolistics by its inventor Sanford (1988). Two types of plant tissue are commonly used for particle bombardment- Primary explants and the proliferating embryonic tissues.
(d) Liposome Mediated Gene Transfer or Lipofection:
Liposomes are circular lipid molecules with an aqueous interior that can carry nucleic acids. Liposomes encapsulate the DNA fragments and then adhere to the cell membranes and fuse with them to transfer DNA fragments. Thus, the DNA enters the cell and then to the nucleus.
(e) Chemical Mediated Gene Transfer:
Chemicals like polyethylene glycol (PEG) and dextran sulphate induce DNA uptake into plant protoplasts. Calcium phosphate is also used to transfer DNA into cultured cells.