Here is a term paper on ‘Biotechnology and Agriculture’ for class 8, 9, 10, 11 and 12. Find paragraphs, long and short term papers on ‘Biotechnology and Agriculture’ especially witten for school and college students.
Term Paper on Biotechnology and Agriculture
Term Paper Contents:
- Term Paper on the Role of Biotechnology in Indian Agriculture
- Term Paper on the State of Indian Agriculture
- Term Paper on the Behaviour of Yields in Major Food Crops
- Term Paper on Agricultural Biotechnology
- Term Paper on the Private Sector R&D in Agriculture
- Term Paper on Gene Technology in Indian Agriculture
- Term Paper on the Regulatory Administration of Biotechnology
Term Paper # 1. Role of Biotechnology in Indian Agriculture:
Biotechnology has been seen as contributing to the development of Indian agriculture. Although policy pronouncements by the Government in this regard have been relatively few, those that have been made need to be underlined. One of the most significant statements about the role of biotechnology in furthering the fortunes of Indian agriculture was made in the National Agricultural Policy presented in the year 2000.
The National Agricultural Policy, which presented the blue-print for the agricultural sector for the next two decades, explored the options to ensure that growth of the sector is sustainable technologically, environmentally and economically. Biotechnology was seen as one of the alternatives for achieving this objective.
The policy stated that the use of biotechnologies would be promoted for evolving plants that are drought resistant, pest resistant, consume less water, contain more nutrition, give higher yields and are environmentally safe.
The Department of Biotechnology (DBT) has complemented this initiative by policy makers in the agricultural sector by advancing their justification for the use of biotechnology for agricultural growth. According to DBT, the post-Green Revolution era has almost merged with the gene revolution for improving the productivity and quality of crops.
The exploitation of heterosis vigour and the development of new hybrids including apomixis, genes for abiotic and biotic resistance, and development of planting material with desirable traits and genetic enhancement of all important cops will be the focus of the agricultural research agenda in the future.
In addition to providing improved quality of plant material, biotechnology has been seen as contributing to integrated nutrient management and development of new bio-fertilisers and bio-pesticides, inputs that would be crucial from the point of view of realizing the objectives of sustainable agriculture, soil fertility and clean environment.
Biotechnology has thus been seen as a key input towards bringing a radical transformation of agricultural practices in India, one that involves a greater use of biological software on a large scale.
The above mentioned objectives that the policy makers have set for biotechnology in the context of transforming Indian agriculture have been reflected in the research priorities set by the DBT in recent years. The Department has been promoting research to enhance food and agricultural production, quality and nutritional improvement and prevention of pre and post-harvest losses.
These research efforts have, according to the DBT, provided significant leads in the areas of basic plant biotechnology and plant genome research, development of makers of high quality protein content and development of molecular methods for hybrid mustard and production of transgenic plants of tobacco with viral resistance.
The focus of agricultural biotechnology as is evident from the policy statements referred to above can be better understood in the context of the structure of Indian agriculture and the imperatives that it faces at the present juncture.
Term Paper # 2. State of Indian Agriculture:
Indian agriculture has been dominated by food crops production. This stems from the basic orientation given to agricultural production by the policy makers, which has been to ensure self-sufficiency in food-grains. Total area under food-grains cultivation was nearly 65 per cent of the gross cropped area in 1997-98.
Although in the subsequent period, the area under food-grains cultivation has decreased somewhat, it nonetheless remains quite significant. Food-grains production is in turn dominated by two of the major cereals, viz., rice and wheat. In 1997-98, these two cereals accounted for 75 per cent of the total food-grains production in the country, which went up to over 77 per cent in 1999-2000.
In terms of the area, rice and wheat accounted for nearly 58 per cent of the total area under food-grains cultivation in 1999-2000. As compared to rice and wheat, maize occupies a relatively minor position in Indian agriculture, accounting for about 3 per cent of the gross cropped area in the late 1990s.
Production of maize has also remained largely unchanged through the 1990s, mainly due to stagnating yields. In sharp contrast, one of the most important non-food crops, viz. cotton does not count for much in terms of the total area under agricultural production.
Although area under cotton production increased during the 1990s, even in 1996-97, the year in which the area under the crop had reached its peak, it was only about 4.5 per cent of the gross cropped area of the country. The overwhelming domination by food-grains in India’s agricultural production can be justified on the grounds that net availability of food-grains in per capita terms has been fluctuating around a declining trend in the country since the mid-1980s.
This trend in per capita net availability cannot be ascribed simply to the changing consumption patterns since per capita GDP of the country has not experienced a dramatic change during the period referred to above.
These figures therefore point to the fact that a country like India, in which poverty in absolute terms remains a major problem, ensuring food security to the population should be the key concern. That considerable efforts need to be put in this direction is evident from the fact that the yields of major crops in India have not only stagnated over a period of time but they are also considerably lower than those observed in some countries.
Term Paper # 3. Behaviour of Yields in Major Food Crops:
In rice the yields have risen steadily but rather slowly over the period 1985 to 1999. In fact, it would not be unreasonable to conclude that yield rates of rice have reached a plateau after the mid-nineties. In 1985, the yield of rice was at 2329 kg/ha.
This increased to 2929 kg/ha in 1999. However, in recent years, the growth rate of yield has declined. The yield increased by 12.8 per cent between 1985 and 1990, after which this rate declined to 1.9 per cent in the period 1990- 95 and further to 5.2 per cent in the period 1995-99.
In case of wheat the yield increased by 13.4 per cent between 1985 and 1990 and further to 20.6 per cent between 1990 and 1995 but thereafter between the period 1995 and 1999 it declined to 0.9 per cent. The yield of wheat was 1870 kg/ha in 1985. It increased to 2559 kg/ha in 1995 and thereafter fluctuated. In 1999 the yield was 2583 kg/ha.
Thus the peak was reached in 1997 after which the yields have declined. Yield of maize, like that of wheat, reached its peak in 1997. Maize recorded a satisfactory growth rate in yield of about 33 per cent during the period 1985 and 1990.
This rate however fell to -2.8 per cent during the period 1990-95, after which it became 0.9 per cent during 1995-99. Yield of maize increased from 1146 kg/ha in 1985 to 1524 kg/ha in 1990 but then it fell to 1481 kg/ha in 1995 and further to 1408 kg/ha in 1996. In 1997 the yield rose to 1746 kg/ha after which it kept falling till 1999. In case of pulses taken as a whole the yields increased steadily from 519 kg/ ha in 1995 to 628 kg/ha in 1995 after which it kept fluctuating till the latest year for which data has been provided.
The yield increased by 7.7 per cent in between 1985-90 and then by 12.3 per cent in between 1990-95. This rate however declined significantly to 0.9 per cent during the period 1995-99. As in case of both wheat and maize, the yield of pulses in 1999 was nearly the same as in 1997, which suggests that no improvement in yield has taken place since 1997.
Yield of rapeseed grew steadily from 771 kg/ha in 1985 to 1017 kg/ha in 1997, which was the peak for the period considered here. Thereafter it fell to 668 kg/ha in 1998 and again increased to 875 kg/ha in 1999. The growth rate of yield during the period 1985-90 was 7.8 per cent. This increased to 13.7 per cent in between 1990-95 and then became negative during the period 1995-99.
The brief trends in yields of some of the major food crops described above indicates that India’s food-grains production strategy needs to focus on ways to improve the yields for the crops included in the analysis. This needs to be the very first step that the country should take to improve agricultural productivity in the foreseeable future.
The yield rates observed in case of India becomes even starker when the figures are compared to those in select countries. The following analysis seeks to compare the yield of two very important cereals; viz. rice and wheat, along with that of maize across countries over the years to throw light on the nature of improvements in yield that have taken place elsewhere.
i. Rice:
India’s rice yields do not compete favorably with the countries figuring in Table 1. Not only were the Indian figures lower than those observed for Sri Lanka, but in the case of China registered yields were more than double than that of India. Countries like Argentina and Brazil, in whose agricultural production rice does not figure prominently, also registered yields that were higher than India had ever recorded.
The only positive aspect for India has been that the rice yields have registered continuous increase between 1985 and 1999, albeit slowly, while for most of the countries appearing in Table 1, rice yields have fluctuated in the same period.
ii. Wheat:
Wheat yields recorded by India compare favorably with those observed for several countries included in Table 2. Only China and the United States have recorded yields that have been higher than those recorded by India. For most countries, however, the wheat yields have peaked during the second half of the 1990s before declining in the closing years of the decade. India also follows this trend, with the highest yield being registered in 1997.
iii. Maize:
Maize yields in India have been among the lowest in the world. Among the countries included in Table 3, only Sri Lanka has lower figures than India. As in the case of the other two food crops discussed above, China and the United States have consistently recorded the highest yields in maize among the countries included in, Table 3.
India’s maize yields in 1999 were almost a third that of China and a fifth that of the United States. In fact during the 1990s, maize yields in India registered only a nominal increase, which does not compare favourably with most other countries included in Table 3.
The above analysis points to the need for a major transformation as far as Indian agriculture is concerned so as to make the sector meet the objectives that have been set by the policy makers.
The imperative has increased given the threat of foreign competition that the domestic producers in India face in the era of open markets. This is an area where technological improvements brought about through the introduction of biotechnology can play a part.
While the effect of structural impediments in depressing production and productivity of the country’s agriculture cannot be ignored, technological solutions can make a contribution in reversing the trends. The success of the Green Revolution Strategy in taking Indian agriculture to a substantially higher growth trajectory provides support to this argument.
Term Paper # 4. Agricultural Biotechnology:
The likely scenario in relation to agricultural products based on biotechnology is presented in Table 5.
The expected growth of consumption indicated by the rising number of seed varieties that are likely to be consumed by the middle of the present decade suggests that agriculture is going to be another important area for large future investment. The seed industry alone could invest over Rs.1.5 billion in the next five years.
Opportunities also exist for new investment in bio-fertilizers (over Rs.200 million), bio-pesticides (about Rs.300 million), pheromones, growth stimulants/promoters (over Rs.500 million) and botanical pesticides.
Trends in Agricultural Research:
In past few years, agriculture in most of the developing countries has been witnessing the introduction of new products, emerging from the developments in the area of biotechnology.
Biotechnology has held particular promise for these countries since it was maintained that the stagnating productivity levels seen in agriculture in developing countries could be reversed. The relevance of this technology for developing countries has to be seen in the light of the priorities that agro-biotech research has seen thus far.
The emergence of the new technologies, biotechnology in particular, has, however, posed serious policy constraints for the developing countries. The international environment in which these technologies are being developed is considerably different from the one which saw the adoption of the high yielding varieties that heralded the advent of the Green Revolution more than three decades ago.
The most significant difference is that unlike the Green Revolution varieties, which were primarily developed in the public-funded organisations, firms in the private sector are spearheading developments in biotechnology.
Avramovic argues that this change in the respective involvement of the institutions has been caused by two factors:
(i) Declining public funding for research, and
(ii) Capital intensive nature of the R&D activity.
The decline, often in relative terms, of public funding for research has taken place essentially because of, the fiscal crisis that the state has been going through in most developing countries.
The larger involvement of the private sector, however, portends to a major change in the structure of agricultural production in developing countries such as India, where enhancing the production of food-grains was set as one of the principal objectives. The direction of public sector research was in keeping with this objective: both food crops and commercial crops formed a part of the research agenda.
But while public sector research took a balanced view, the private sector has shown a keen interest in the commercial crops, giving very little attention to the crops linked with the food security of a developing country. In fact, such crops are now being called ‘orphan crops’, which are primarily consumed by small and marginal farmers and by the agricultural labourers.
Further, as part of their globalization efforts, many developing countries have been encouraging foreign investment in food processing industries, which has further taken away the focus of the R&D priorities from the primary crops to the commercial crops, a point that will be elaborated below.
This approach towards R&D raises many questions regarding access to biotechnology which is appropriate to national needs. The issue of food security is inextricably linked to the growth of technology in this sector, and that agriculture continues to provide livelihoods to a majority of the population.
Term Paper # 5. Private Sector R&D in Agriculture:
Agricultural research in India has three distinct components. The first component of research is the development of the high yielding varieties, which have been popularized after the Green Revolution. The second component is production of hybrids, while the third is the production of genetically modified seeds.
The involvement of the private sector in R&D in the agricultural sector has traditionally been at a very low level. As in the case of most countries, India has seen an overwhelmingly large participation by government- funded organisations in agricultural research until the end of the 1990s. Production of the high yielding varieties, in particular, was in the hands of the public sector organisations, which include the agricultural universities, set up with state support.
The participation of the private sector was by only a few firms, which had started operating in the 1960s. These firms concentrated their efforts on developing superior hybrids. By 1980s, the number of private sector seed firms had gone up to just six, and by the end of the decade of the ’80s, private sector seed firms were only 12.
One of the principal factors restricting private sector participation in the seed sector until the end of the 1980s was the restrictions imposed by the government on their expansion through the use of the licensing policy. The provisions of the licensing policy as applicable to the production of hybrid seeds and agricultural biotechnology products were relaxed in 1987.
Foreign firms and firms belonging to the monopoly houses, which were, till then, prevented from investing in the seed industry, were now given permission to do so.
Further encouragement to the growth of the private sector seed industry in India was given in 1988. Import of seeds of coarse cereals, pulses and oilseeds was allowed for a period of two years by firms established in India which had entered into collaboration with foreign firms for the production of seeds, provided that the foreign partners agreed to supply the parent-lines of the seeds to the Indian partners within two years from the date of import of the first commercial consignment. This policy excluded wheat and paddy mainly because of the strong presence of the public funded organizations in the production of these two crops.
The import of seeds and/or planting material of fruits, vegetables, flowers and ornamental plants were freed from government control in 1989, in accordance with the provisions of Plants, Fruits and Seed Order of 1989. The major involvement of the private sector in the seed industry has been through the production of hybrids. Development of genetically engineered seeds is at best in its early days.
The developments in the two segments of the Indian seed industry are discussed below:
Private Sector in the Production of Hybrids:
A better indicator of the growth of the private sector in the seed industry in India is the increase in the share of this sector in the volume of seed sales. In three of the six crops, almost the entire domestic production during 1996-97 was accounted for by the private sector firms.
Maize and sunflower have in fact witnessed a supplanting of the public sector by the private sector. As compared to the volume of production, the share of the private sector seed business in the total value of production of the major crops during 1996- 97 have been somewhat higher.
The above discussion shows that private sector R&D was restricted to crops other than the two main cereals consumed in India, viz. rice and wheat. Rice is produced on nearly 42 million hectares and given this large market coupled with the fact that there is need to provide new varieties to improve productivity, private sector firms have begun making investments in R&D in developing rice hybrids.
Term Paper # 6. Gene Technology in Indian Agriculture:
These research efforts, according to the DBT, have provided significant leads in the areas of basic plant biotechnology and plant genome research, development of makers of high quality protein content and development of molecular methods for hybrid mustard and production of transgenic plants of tobacco with viral resistance.
The focus of agricultural biotechnology, as is evidenced from the policy statements referred to above, can be better understood in the context of the structure of Indian agriculture and the imperatives that it faces at the present juncture.
The application of the gene technology can be brought about in three ways:
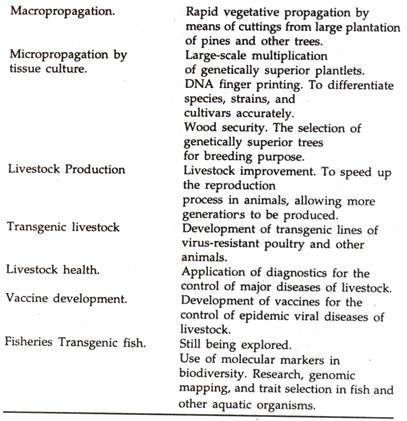
i. By selection of improved varieties through genome mapping to identify and propagate high yielding cultivars and utilization of anther/pollen culture to speed up propagation of high yielding varieties.
ii. Developing cultivars resistant to viruses, bacteria, fungi and pests tolerant to herbicides, salinity, drought, and heat water-logging, among other problems affecting production and productivity.
iii. Improving the economic value of existing products: by delaying the ripening of fruits to improve their shelf life or by modifying cotton cultivars to improve their fibre qualities.
Of the applications that have been indicated above, imparting specific insect-pest resistance through the transfer of genes from Bacillus thuringiensis (Bt) into target plants is considered as one of the most advanced applications of biotechnology at present. These Bt genes that characterise different crystalline proteins are toxic to certain insect-pests.
The Bt proteins selectively act on the insect-pests like caterpillars, beetles, flies and mosquitoes and are thus considered key to presenting crop losses due to pest infestation.
Proponents of biotechnology have argued that the use of Bt gene would have two major advantages. First, it would help provide an effective guard to the crops against the insect-pests and prevent major damage to the crops.
Secondly, and more importantly, this manner of preventing crop losses would be safer and more cost effective than the conventional method of countering pest infestation using pesticides. By replacing the toxic pesticides, Bt gene can help protect the farmers, the consumers and, above all, the environment.
Genetic engineering in the Indian seeds industry is in its early days for most of the ongoing research is presently limited to a few crops and to specific problems related to these crops. Some of the more significant attempts to genetically modify seeds.
One of the key problems that genetic engineering is trying to address is the excessive use of chemical pesticides and insecticides by the farmers on vegetables since most of the insects of these crops are developing resistance to these chemicals.
The joint venture of Maharashtra Hybrids (MAHYCO) Monsanto is testing a genetically modified cotton variety that is resistant to insects. Similarly, Rallis India is also working on various vegetable crops to introduce insect resistance based on the technology acquired from aboard.
Another leading company in India that is involved in the development of transgenics is ProAgro Seed Company Private Ltd., which is working on crops such as Indian mustard, cauliflower, cabbage, tomato and eggplant to introduce genes of insect resistance and male sterility. At present, in India there are no hybrids in Brassica Juncea and the yields have not been increasing for many years and all efforts to produce hybrids through natural male sterility systems have not been successful.
The major cause for this is the fact that the attempts to introduce these seeds have come at a time when considerable debate is taking place globally on the likely adverse implications of the genetically modified organisms (GMOs) on the environment in general and plant, animal and human health in particular. The global concern has also triggered off some debate in India on the effectiveness of the regulatory mechanism in dealing with the GMOs.
Term Paper # 7. Regulatory Administration of Biotechnology:
Biosafety policies in India are governed by the Environment (Protection) Act of 1986. In accordance with this Act, the Rules for the Manufacture, Use/Import/Export and storage of Hazardous Micro-Organisms/Genetically Engineered Organisms or Cells were notified in December 1989.
The rules were made applicable to a set of specific cases, which include:
(a) Sale, offers for sale, storage for the purpose of sale, offers of any kind of handling over with or without a consideration,
(b) Exportation and importation of genetically engineered cells or organisms,
(c) Production, manufacturing, processing, storage, import, drawing off, packaging and repackaging of the genetically engineered products; and
(d) Production, manufacture, etc., of drugs and pharmaceuticals and food stuffs, distilleries, tanneries etc. which make use of microorganisms/ genetically engineered micro-organisms one way or another.
The Rules established the institutional structure for operationalizing the biosafety policies in the country.
A six-tier structure was put in place which had the following components:
(1) Recombinant DNA Advisory Committee,
(2) Institutional Biosafety Committee,
(3) Review Committee on Genetic Manipulation,
(4) Genetic Engineering Approval Committee,
(5) State Biotechnology Co-ordination Committee, and
(6) District Level Committee.
These six bodies were designed to cover the entire decision-making involving research, use and application of biotechnology, as elaborated below. While the first of three Committees function directly under the Department of Biotechnology, the remaining three are linked to the Ministry of Environment and Forests.
(1) Recombinant DNA Advisory Committee:
The Recombinant DNA Advisory Committee (RDAC) has been mandated to review developments in biotechnology at national and international levels and to recommend suitable and appropriate safety regulations for India on recombinant research, use and applications. The RDAC, which functions under the Department of Biotechnology (DBT), has three broadly defined objectives.
These are:
(i) To evolve a long-term policy for research and development involving recombinant DNA;
(ii) To formulate the safety guidelines for recombinant DNA research to be followed in India; and
(iii) To recommend mechanisms for raising awareness among the personnel of the risks and hazards involved in recombinant research. The RDAC is expected to meet at least twice a year.
(2) Institutional Biosafety Committee:
The Rules require every institution in India engaged in research involving genetic engineering and production of genetically engineered products to constitute an Institutional Biosafety Committee (IBSC). The IBSC is required to comprise at least six members including the Head of the Institution or his nominee.
Two of the IBSC members are required to be from outside the Institution. Of the two external members, one would have to be nominated by the DBT and the other a scientist engaged in recombinant DNA research.
The IBSC has been identified as the point for interaction within an Institution for the implementation of safety guidelines adopted in 1990 as well as the revised guidelines for research in transgenic plants adopted in 1998.
Any research project, which is likely to have biohazard potential as indicated by the guidelines, during the execution stage, or which involve the production of either microorganisms on biologically active molecules that could cause biohazard have to be notified to the IBSC. The IBSC has been empowered to allow genetic engineering activity on classified organisms only at places where such activity should be performed according to the guidelines.
Each of the IBSCs is expected to carry out the following functions:
(i) Registration of the membership of the Committees with the Review Committee on Genetic Manipulation (RCGM) of the DBT and submission of half-yearly reports on the on-going projects;
(ii) Review and clearance of project proposals that meet the requirements of the guidelines; and
(iii) Training of personnel and instituting health monitoring programme for them.
At the time when the RCGM formally started functioning in 1993, IBSCs had been established in about 49 institutions. By the second half of 1998, IBSCs had been set up in 124 institutions in India.
In 2000-2001 the number of institutions having IBSCs had increased to 150 while other institutions, according to DBT, were being asked to take the requisite steps to set up these Committees.
(3) Review Committee on Genetic Manipulation:
The Review Committee on Genetic Manipulation (RCGM) is one of the key elements in the biosafety infrastructure that is in place in India. RCGM was established by the DBT in 1993 to monitor the safety related aspects of the on-going research projects and activities involving genetically modified organisms/hazardous microorganisms.
The RCGM includes representatives from the Indian Council of Medical Research (ICMR), the Indian Council of Agricultural Research (ICAR) and the Council of Scientific and Industrial Research (CSIR). These organisations are statutory bodies of the Government of India engaged in research in identified areas. Besides, the RCGM includes experts who participate in the Committee meetings in their individual capacity.
In 1998, following the adoption of the revised guidelines for research in transgenic plants, the RCGM established a Monitoring-cum-Evaluation Committee (MEC) to monitor the impact of transgenic plants on the environment. Included in this Committee were seed technologists and plant breeders nominated by ICAR, a representative of the Ministry of Environment and Forests besides plant biotechnologists and plant ecologists nominated by the RCGM.
The MEC was mandated to undertake field visits at the experimental sites where transgenic plants were being tested and provide data relating to the trials that could be used for evaluating the environmental risks emanating from transgenic plants. This Committee was expected to advise the RCGM on the risks and benefits arising out of the use of transgenic plants whose trials it would be monitoring.
Field trials were to be done for at least one year with a minimum of four replications and ten locations in the agro- ecological zone in which the plants were to be grown. The MEC was authorized to recommend those transgenics, which were found to be environmentally viable by the RCGM, to the Genetic Engineering Approval Committee under the Ministry of Environment and Forests for consideration for release into the environment.
The RCGM was established on the basis of the 1990 guidelines for research on transgenic plants. The guidelines were amended first in 1994 and subsequently in 1998.
The RCGM was assigned the following functions in the 1990 guidelines:
(i) To establish procedural guidelines for regulating activities involving genetically engineered organisms in research, production and application related to environmental safety;
(ii) To review the reports in all approved on-going research projects involving high risk and controlled field experiments in order to ensure that safeguards were maintained in keeping with the guidelines;
(iii) To recommend the type of containment facilities and special containment conditions that were required to be followed for experimental trials and other experiments;
(iv) To advise the customs authorities on import of biologically active material, genetically engineered substances or products, and on excisable items to Central Revenue and Excise;
(v) To assist the Department of Industrial Development and Financial Institutions for clearance of applications for setting up industries based on genetically modified organisms;
(vi) To assist the Bureau of Industrial Standards to evolve standards for biologics produced by recombinant DNA technology; and
(vii) To advise on intellectual property rights with respect to recombinant DNA technology.
The RCGM was clearly conceived of as a regulatory authority that was to link up all the different activities that involve the use, production and application of genetic engineering as a technology.
The regulatory functions of the RCGM were strengthened further in the revised guidelines for research in transgenic plants and guidelines for toxicity and allergenicity evaluation of transgenic seeds, plants and plant parts adopted in 1998.
At the same time, however, RCGM was sought to be re-positioned as a facilitator in the efforts to effectively develop biotechnology in the country. Introducing at least two additional dimensions strengthened the regulatory role.
First, the revised guidelines provided a new classification of risks associated with experiments involving transgenic plants. Among the major changes that were effected in this respect was that it was made mandatory for all projects involving recombinant DNA technologies to at least inform the IBSC of the details involved.
This was a departure from the 1990 guidelines, which exempted projects that were generally considered as safe to humans, animals and plants from giving any intimation to the RCGM about the activities involved.
The second major change in respect of classification of Category III risk which included transgenics that can cause alterations in the biosphere. All open field experiments of transgenic plants, howsoever organised, have been included in Category III risk.
Thus, even when experiments are conducted under reasonably contained conditions by taking all the precautions to prevent the escape of transgenic plants or their parts that have propagating traits, seeds.
Secondly, the RCGM, assumed the powers to direct the applicants to generate data pertaining to:
(a) Toxicity allergenicity and any other relevant data on transgenic material;
(b) Long term environmental safety; and
(c) Economic advantages of the transgenic over the existing varieties.
The facilitating role of RCGM towards promoting research in genetic engineering involving plants was seen on two counts.
These were:
(i) RCGM could issue clearances for import/export of seeds and plant parts and other material required for conducting research; and
(ii) RCGM could authorize limited field trials in multi- locations in the country.
The design of the trial experiments could be provided either by the RCGM or it could approve the protection designed by the applicants. These guidelines adopted in 1998 underlined the detailed procedures for conducting contained field experiments using transgenic plants.
The contained field experiments are to be conducted in a manner that can arrest the escape of transgenic plants or plant parts, including seeds, into the open environment. In addition, these experiments are also designed to create a reasonably effective barrier to prevent the escape of pollen from the transgenic plants into the environment.
RCGM monitors research on transgenic organisms in the laboratory and in the contained open environment and fields. In the case of transgenic plants, experiments are conducted in contained green houses to generate vital safety information before decisions are taken to conduct contained open field trials. Through these trials, RCGM tries to obtain information on environmental safety, including human and animal food safety issues for all kinds of transgenics.
(4) Genetic Engineering Approval Committee:
The Genetic Engineering Approval Committee (GEAC) was constituted under the Ministry of Environment and Forests (MOEF) for approval of activities involving large-scale use of hazardous microorganisms and recombinants in research and industrial production.
The GEAC was also identified as the agency responsible for approval of proposals relating to the release of genetically modified organisms and products into the environment, including experimental field trials.
With its focus on the protection of the environment, the GEAC was mandated to monitor the following activities:
(i) Import, export, transport, manufacture, processing, selling of any micro-organism or genetically engineered substances or cells including food stuffs and additives;
(ii) Discharge of genetically engineered organisms/cells from laboratories, hospitals etc., into the environment;
(iii) Large scale use of genetically engineered organisms in industrial production and applications; and
(iv) Deliberate release of genetically modified organisms.
The membership of GEAC covered the widest spectrum of Government agencies. Four Ministries/Departments were represented on the Committees. These were: Ministry of Industrial Development and Departments of Science and Technology, Ocean Development and Biotechnology.
Other members of GEAC were representatives from ICAR, ICMR, CSIR, Central Pollution Board and Health Services, the last named being under the Ministry of Health and Family Welfare. Among the other members of GEAC were the Plant Protection Adviser and three experts in the relevant fields. More recently, the objectives of GEAC have been fine-tuned.
According to these revised set of objectives, GEAC was expected to issue clearances from the point of view of environmental safety on a case-by-case basis for:
(i) Activities involving large scale use of hazardous microorganisms and recombinants in research and industrial production from an environmental angle;
(ii) Proposals relating to the release of genetically engineered organisms and products into the environment including field trials;
(iii) Production, sale, import or use of substances and products including food stuffs and additives including processing aids containing or consisting of genetically engineered organisms or cells or micro-organisms;
(iv) Import, export, transport, manufacture, process, use or sale of any hazardous micro-organisms or genetically engineered organisms/ substances or cells; and
(v) Scale up or pilot operations for facilities using genetically engineered organisms/micro-organisms.
Alongside the fine-tuning of its objectives, the membership of GEAC was expanded by involving a number of additional ministries. The Ministries of Commerce and Industry, Food Processing Industries, Health and Family Welfare and External Affairs and the Department of Agriculture were included in the GEAC.
(5) State Biotechnology Coordination Committee:
The State Biotechnology Coordination Committees (SBCCs) were conceived in keeping with the federal structure of state polity that India has developed. These Committees assume further significance in the context of the sharing of responsibilities between central government and the state in the crucial area of agriculture, which, according to the Indian constitution, is to be managed entirely by the state government.
The SBCCs were given the powers to inspect, investigate and take punitive action in case of violations of statutory provisions under the Environment (Protection) Act, 1986.
More specifically those Committees were assigned the following functions:
(i) Review and control safety measures adopted while handling large scale use of genetically modified organisms in research, developmental and industrial production activities;
(ii) Monitor large scale release of genetically engineered products with the environment, and oversee field applications and experimental field trials; and
(iii) Provide information/data to RCGM upon surveillance of approved projects, and in case of environmental releases, with respect to safety, risks and accidents.
The members of the SBCCs included representatives from the state Ministries of Environment, Health, Agriculture, Industry and Forests. The State Pollution Control Board and microbiologists from the state were the other prominent members.
(6) District Level Committee:
The Rules of the Environment (Protection) Act provides for the establishment of the District Level Committees (DLCs) wherever necessary to monitor the safety regulations in installations engaged in the use of genetically modified organisms. The DLC could impact any installations engaged in activities involving genetically modified organisms and identify the sources of risks associated with such installations and coordinate activities with a view to meeting any emergency.
The DLC was expected to submit regular reports to the relevant SBCC and GEAC. As in the case of the other Committees, the members of the DLC were government officials who were involved in the areas of agriculture, pollution control arid health.
The Committees that form the regulatory administration of biotechnology in India have clearly marked out functional areas as was elaborated above. However, the manner of their functioning does leave some room for doubting, at least on two counts, whether or not the tasks assigned to them are effectively accomplished.
First, the nature of intervention of the State Biotechnology Coordination Committees and the District Level Committees in the regulatory process is not clear and this arises from the absence of the requisite information. In other words, although a decentralized structure has been provided for carrying out the regulatory functions, no real attempt seems to have been made to make it respond to the problem at hand.
The second issue which is related to the first is the low level of transparency that the regulatory administration maintains. This was quite in evidence in the process leading up to the approval for commercial exploitation of the first genetically modified crop in India.
Status of Regulatory Approvals:
It was indicated that a number of publicly funded institutions and private sector companies have been involved in the development of transgenics involving a range of crops. Most of these crops are commercial crops, which include a range of vegetables, cotton, tobacco and mustard. These crops are in various stages of development and field-testing, after having received the necessary approval of the RCGM.
The genetic modifications that have been carried out in a vast majority of these crops are intended to introduce pest resistance. Yet another focus of the genetic transformations has been the production of higher value hybrid crops such as mustard.
Although development of transgenic has taken off in the country encompassing the private and the public sector, no transgenic crop has yet been granted unrestricted approval for commercial application. Contained field trials have been taking place in case of tobacco, mustard, tomato, brinjal (egg-plant) and cotton.
Of these crops, transgenic cotton being developed by MAHYCO which is resistant to cotton bollworm is the only one to have received approval for limited commercialization by the GEAC. The case of transgenic cotton has become the testing ground for regulatory mechanism for genetically modified crops in India.