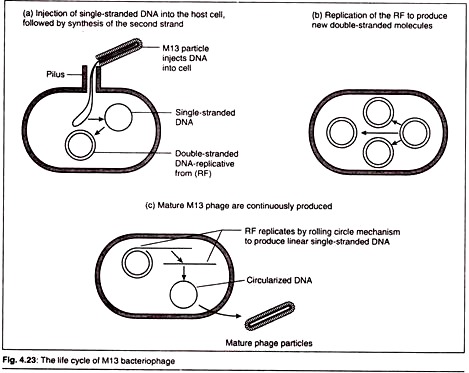
Here is a term paper on the ‘Biomolecules of Life’ for class 8, 9, 10, 11 and 12. Find paragraphs, long and short term papers on the ‘Biomolecules of Life’ especially written for school and college students.
Term Paper on the Biomolecules of Life
Term Paper Contents:
- Term Paper on Water
- Term Paper on Minerals
- Term Paper on Carbohydrates
- Term Paper on Proteins
- Term Paper on Lipids
- Term Paper on Nucleic Acids
Term Paper # 1. Water:
Water is the most important for life to exist and survive. It is present in almost all forms of life forming 60% of volume. Its atomic structure is very simple containing two hydrogen (H) atoms connected to one oxygen (O) atom.
This structure makes it a universal solvent. H has slight positive charge while O has slight negative charge. This also gives strong surface tension. In physical state, water molecules arrange themselves according to mass and density.
In liquid state, water molecules arrange themselves in a connected small groups, which decreases mass and density. In gas, water is highly charged with kinetic energy. This causes it to move with loose bonds among water vapor.
Water has many physical properties, such as high specific heat that helps organisms regulate body temperature. Water has neutral pH which changes according to its solvent. It can be acidic or basic.
Water conducts heat easily which causes large bodies of water to have uniform temperature. In the liquid form, water temperature ranges from 0 °C to 100 °C. As it dissolves many chemical compounds, it is called universal solvent. Hence, water is present in many chemical substances.
The high surface tension and adhesive and elastic nature allows water to form moles droplets and waves. It also allows plants to move water from results to leaves. In animals, it allows to move blood through tiny vesicles. All three phases of water exists on account of temperature change.
Term Paper # 2.
Minerals:
Essential Element:
The essential elements take part in nutrition, growth, development and function of organelles in cells. In the absence of these essential elements, the organisms cannot grow and reproduce. Its deficiency causes disorders. C, H, O are called framework elements as they produce call wall and storage products. C, H, O, N, S and P are called protoplasmic elements as they produce proteins, nucleic acids, etc.
Non-Essential elements:
The non-essential elements are not involved in metabolism, structure or functioning of organisms. Also, there deficiency doesn’t show any disorder. In living beings, minerals occur in two states- free and complex state as component of organic molecules and inorganic molecules or ions. They have balance between complex and free-state depending on the concentration required, essential minerals are major or minor.
Term Paper # 3.
Carbohydrates:
Carbohydrates form a very large group of naturally occurring organic compounds. The substances which are formed by bonding C and H are called organic compounds. A carbohydrate molecule contains carbon, hydrogen and oxygen. Carbohydrates are chemically hydrated carbon. The most common carbohydrates are glucose, fructose, sucrose, starch and cellulose.
The carbohydrates are defined as poly hydroxy aldehydes or ketones or substances which give such molecules on hydrolysis. The empirical formula of carbohydrates is Cn(H2O)n (where n is number of atoms). Many carbohydrates are sweet in taste and all sweet carbohydrates are called as sugar. The chemical name of the most commonly used sugar in household food is sucrose.
The carbohydrates (saccharides = sugar) are divided into following three chemical groups:
1. Monosaccharaides:
A carbohydrate which cannot be hydrolyzed further to a smaller molecule containing these functional groups is known as a monosaccharide. They have very simple structure. About 20 monosaccharaides occur in nature and glucose is the most common one. They are subdivided into trioses, tetroses, pentoses, hexoses and heptoses depending on carbon atoms they have and if they are aldoses or ketoses.
Examples:
Examples of monosaccharaides are glucose (probably the most abundant organic compound on the earth), D-Fructose (a ketohexose – sugar that is found with glucose in honey and fruit juices), and glyceraldehyde.
2. Disaccharides:
Two composed monosaccharaides are called as disaccharide. These are the simplest polysaccharides. Examples include sucrose and lactose. They are composed of two monosaccharide units bound together by a covalent bond known as a glycosidic linkage.
Sucrose is the most abundant disaccharide, and the main form in which carbohydrates are transported in plants. It is composed of one D-glucose molecule and one D-fructose molecule. Lactose is a disaccharide composed of one D-galactose molecule and one D-glucose molecule. It occurs naturally in mammalian milk.
3. Polysaccharides:
If a large number of monosaccharide units are joined together, a polysaccharide is formed. These are the most common carbohydrates found in nature. They have mainly one of the following two functions- either as food materials or as structural materials of the cell or body.
Starch is the main food storage polysaccharide of plants. It is a polymer of α-glucose and consists of two types of chains- known as amylose and amylopectin. Starch consists of a large number of glucose units joined together by glycosidic bonds.
Starch is produced by all green plants as an energy store. It is the most important carbohydrate in the human diet and is contained in such staple foods as rice, wheat, maize (corn), potatoes and cassava.
When all the monosaccharaides in a polysaccharide are of the same type, the polysaccharide is called a homopolysaccharide or homoglycan, but when more than one type of monosaccharide is present in a polysaccharide, it is called heteropolysaccharide or heteroglycan.
Glycogen is also called as animal starch. It is densely branched polymer of glucose. In a polysaccharide chain (say glycogen), the right end is called the reducing end and the left end is called the non-reducing end.
Cellulose and chitin are examples of structural polysaccharides. Cellulose is present in the cell walls of plants and other organisms, and is said to be the most abundant organic molecule on earth.
It has many uses such as a significant role in the paper and textile industries. Chitin has a similar structure, but has nitrogen containing side branches, increasing its strength. It is found in arthropod exoskeletons and in the cell walls of some fungi.
Significance of Carbohydrates:
They act as biofuels to provide energy for functioning of organisms and also as constituents of cell membranes.
Term Paper # 4.
Protein:
Proteins are the most abundant macromolecules in living cells. The name protein is derived from the Greek word ‘proteios’ meaning ‘of prime importance’. These are high molecular mass complex polymers of amino acids.
A living system contains thousands of different proteins for its various functions. In our everyday food pulses, eggs, meat and milk are rich sources of proteins and are must for a balanced diet. Fibrous Proteins are long thread like protein molecules.
They are generally water-insoluble. Examples are collagen (major protein of connective tissues), elastin (protein of arteries and elastic tissues), and keratins (proteins of hair, wool, and nails). Globular proteins are folded into compact units which are spherical in shape. Examples are albumin of eggs, globulin (present in serum), and haemoglobin.
Amino Acid:
Protein molecules are polymers of different sizes and shapes with different physical and chemical properties. The monomer units for proteins are amino acids. An amino acid is a molecule that contains both amino (NH2) and carboxyl (COOH) functional groups. Alanine is one of the standard amino acids. There are 22 amino acids which constitute the proteins due to polymerization.
Certain amino acids are essential for our health and they have to be supplied through our diet. The dietary proteins are the source of these essential amino acids. Therefore, amino acids can be essential or nonessential. The non-essential amino acids are those which our body can make, while we get essential amino acids through our diet/food.
Collagen is the most abundant protein in animal world and Ribulose bisphosphate Carboxylase- Oxygenase (RuBisCO) is the most abundant protein in the whole of the biosphere.
Polypeptide:
A linear chain of amino acid residues is called a polypeptide. All the proteins are polypeptides.
Protein:
A protein contains at least one long polypeptide.
Peptides:
Short polypeptides, containing less than about 20-30 amino acids, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides.
Peptide Bond:
The individual amino acid residues are bounded together by peptide bonds and adjacent amino acid residues. In peptide bond, an amide bond is formed when the carboxyl group of one amino acid molecule reacts with the carboxyl group of another. In this process, a molecule of water is given off.
Protein:
Peptides formed by the combination of more than ten amino acid units are called polypeptides. Proteins are polypeptides formed by the combination of large number of amino acid units. Example insulin contains only 51 amino acids, but is generally considered a small protein.
Significance of Proteins:
Proteins are basis for protoplasm and are present in all living organisms in muscle, hair, skin, as enzymes and hormones. Also some of them are antibodies for protection of organisms.
Term Paper
# 5. Lipids:
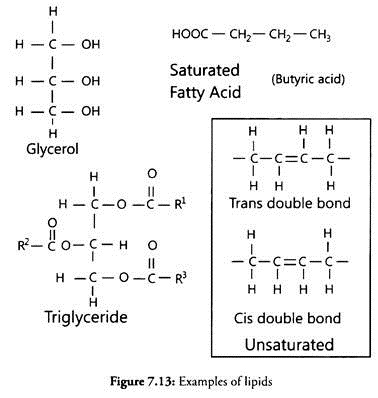
Lipids are generally water-insoluble molecules. They are simple fatty acids. A fatty acid has a carboxyl group attached to an R group which could be a methyl (-CH3), or ethyl (-C2H5) or higher number of-CH2 groups (1 carbon to 19 carbons).
For example, palmitic acid has 16 carbons including carboxyl carbon. The arachidonic acid has 20 carbon atoms including the carboxyl carbon. Fatty acids could be saturated (without double bond) or unsaturated (with one or more C=C double bonds). Another simple lipid is glycerol which is trihydroxy propane.
Many lipids have both glycerol and fatty acids. Here, the fatty acids are found esterified with glycerol. They can be monoglycerides, diglycerides and triglycerides. These are also called fats and oils based on melting point.
Oils have lower melting point (e.g., gingili oil) and hence remain as oil in winters. Some lipids have phosphorous and a phosphorylated organic compound in them. These are phospholipids. They are found in cell membrane, for example, lecithin. Some tissues especially the neural tissues have lipids with more complex structures.
Term Paper
# 6. Nucleic Acids:
It was recognized in the 19th century, that the nucleus of a living cell contains particles responsible for heredity, which were called chromosomes. These are composed of nucleic acids. Nucleic acid comes from the nucleus of the cell.
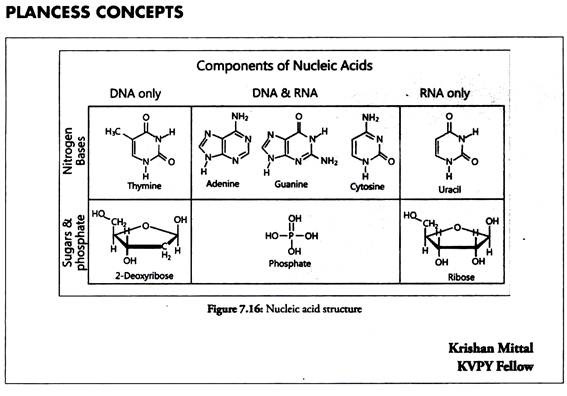
These are acidic in nature and thus named Nucleic acids. There are two types of nucleic acids which are called DNA (deoxyribonucleic acid) and RNA (ribonucleic acid). They differ in their chemical composition as well as in functions.
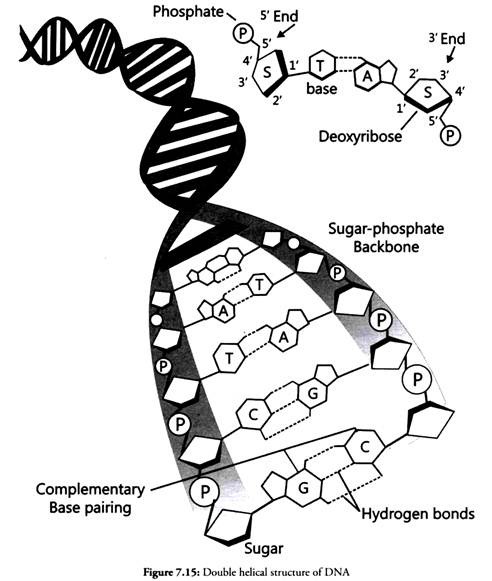
Nucleic acids are chain like polymers of thousands of nucleotide units. Thus, they are also called polynucleotides. A nucleotide consists of three subunits- nitrogen containing heterocyclic aromatic compound (called base), a pentose sugar and a molecule of phosphoric acid.
In DNA molecules, the sugar moiety is 2-deoxyribose, whereas in RNA molecules it is ribose. In DNA, four bases have been found. They are adenine (A), guanine (G), cytosine (C) and thymine (T). The first three bases are common in RNA. The fourth base found in RNA is uracil (U).
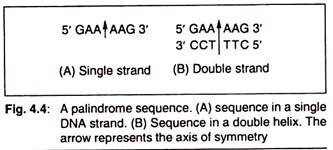
Nucleic acids exhibit a wide variety of secondary structures. For example, one of the secondary structures exhibited by DNA is the famous Watson – Crick Model. As per this model, the DNA is a double helix.
The two strands of polynucleotides are antiparallel i.e., run in the opposite direction. The backbone is formed by the sugar-phosphate-sugar chain. The nitrogen bases are projected more or less perpendicular to this backbone but face inside.
A and G of one strand compulsorily base pairs with T and C, respectively, on the other strand. There are two hydrogen bonds between A and T and three hydrogen bonds between G and C. Each strand appears like a helical staircase. Each step of ascent is represented by a pair of bases.
At each step of ascent, the strand turns 36°. One full turn of the helical strand would involve ten steps or ten base pairs. The pitch would be 34Å. The rise per base pair would be 3.4Å. This form of DNA with the above mentioned salient features is called B-DNA.