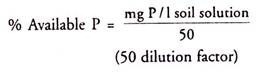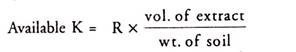In this article we will discuss about the estimation of phosphorus and potassium for analysing soil.
Estimation of Available Phosphorus:
i. Olson’s Method:
Principle:
Available phosphorus is extracted from soil with N/2 sodium bicarbonate with pH adjusted to 8.5. By adding molybdate and stannous chloride solution a blue colour develops and this is read on a photo electric colorimeter.
Requirements:
Reagents:
1. Bicarbonate extractant:
840 g sodium bicarbonate in 20 litres distilled water. Add NaOH/HCl and adjust pH to 8.5, filter.
2. Activated carbon:
Dacro G60 or any suitable decolourising carbon made free from soluble phosphorus by repeated washings with bicarbonate extracting solution.
3. Molybdate reagent:
Dissolve 1.50 g of ammonium molybdate in 300 ml distilled water. Add the solution to 400 ml of 10(N) HCl gradually with stirring. Dilute to one litre with distilled water (note: if the concentration of HCl used is not exactly 10 (N) add calculated volume of ‘ the acid equivalent to 400 ml of 10(N) HQ.
4. Stannous chloride:
Dissolve 10 g SnCl2. 2H20 in 25 ml conc. HCl. Add a piece of pure metallic tin and store the solution in a glass stoppered bottle.
5. Working solution:
Dilute 1 ml of stock solution to 66 ml with distilled water just before use.
6. Photoelectric colorimeter.
7. Multiple dispensor or automatic pipette.
8. Bulb pipettes.
9. Conical flasks.
10. Shaker.
11. kH2PO4.
12. 25 ml measuring flasks.
13. Soil sample.
14. Distilled water.
Procedure:
1. Preparation of standard phosphorus:
Dissolve 0.1916 g of pure KH2PO4 in one litre of distilled water. This solution contains 0.10 mg P2O5 per ml. Preserve this with a drop of toluene. Take 10 ml of this solution and dilute to 1000 ml with distilled water. This solution contains 0.001 mg of P2O2/ml. Take 1,2,4,6 and 10 ml of this solution in separate 25 ml measuring flasks.
2. Extraction of soil:
Take 11, 100 ml conical flasks and add 50 ml of the bicarbonate extractant in each flask having 2.5 g soil sample. Add 1 g of decolourising carbon (activated carbon) shake for 30 minutes on a mechanical shaker and filter.
3. Take 5 ml of the filtered soil extract with a bulb pipette in a 25 ml measuring flask.
4. Add 5 ml of molybdate reagent to the soil extract as well as to the series of standard solutions in 25 ml measuring flasks.
5. Make up the volume to 20 ml in all flasks with distilled water, shake and add 1 ml of dilute SnCl2 solution with a bulb pipette to all the flasks.
6. Fill to 25 ml mark with distilled water and shake well.
7. Read the blue colour which develops after 10 minutes on the photoelectric colorimeter using 660 millimicron red filter after setting the instrument to zero with blank prepared without standard or soil extract.
8. Plot a graph of standard concentration against colorimeter readings and find out the concentration of P2O5 of unknown (soil) from it.
ii. Trivedi and Goel:
Principle:
This method gives approximate values. Phosphorus in soil is generally determined as available phosphorus which can be extracted from soil with 0.002 (N) H2S04 (soil: H2SO4= 1:200 V/V).
Requirements:
1. Reagents:
H2SO4 (0.002 N), Dilute concentrated H2SO4, 360 times (2.78 + 1000 ml distilled water)-stock solution. Dilute suitable quantity of stock solution 50 times to give 0.002 (N) H2SO4 Make final pH 3.0 by adding 3 g (NH2) SO4 or K2SO4.
2. Ammonium molybdate solution: 25 g of ammonium molybdate in 175 ml distilled water.
3. Stannous chloride: 2.5 g. in 100 ml glycerol, heat on a water bath for rapid dissolution.
4. Standard PO4: Dissolve 4.388 g of dried anhydrous K2HP04 in distilled water and make up the volume to one litre. Dilute a portion of this solution 100 times using distilled water. This standard PO4 solution has 10 mg P/l (1 ml=0.01 mg P).
5. Soil sample, activated charcoal.
6. Flasks, filter papers.
7. Shakers.
8. Spectronic 20.
Procedure:
1. Sieve soil through 2 mm screen and determine its moisture content by drying it overnight.
2. Take 10 g fresh soil on a dry weight basis in 500 ml flask and add 200 ml of 0.002 (N) H2S04. Keep on shaker at 1200 rpm for 30 minutes and filter suspension.
3. Take 50 ml of clear filtrate in a clean conical flask. If filtrate is coloured, add little activated charcoal, filter and use.
4. Add 2 ml of ammonium molybdate followed by 5 drops of SnCl2).
5. Take O.D. at 690 nm of the blue colour developed along with blank (D.W + same amount of ammonium molybdate+5 drops SnCl2).
6. Take readings after 5 minutes, but before 12 minute of addition of last reagent.
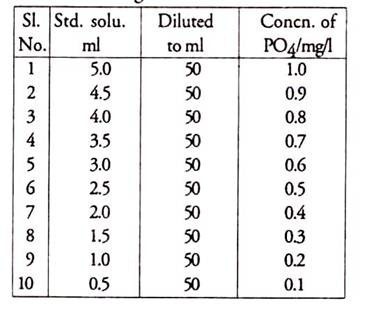
7. Prepare a standard PO4 curve as shown in the following table:
Take 50 ml of each dilution + 2 ml ammonium molybdate and 5 drops of SnCl2. Take reading at 590 nm and plot a graph with absorbance and concentration of P from this.
Calculation:
Estimation of Potassium:
Requirements:
Reagents:
1. Neutral normal ammonium acetate. Mix equal volumes of 2 (N) acetic acid and 2 (N) ammonium hydroxide and adjust pH to 7.0 with acid or ammonia.
2. KCl.
3. Flame photometer.
Procedure:
1. Shake 5 g of soil with 25 ml neutral normal ammonium acetate (pH 7) for 5 minutes.
2. Filter immediately through filter paper. Reject first few ml of this filtrate. Calibrate the flame photometer and estimate standard K curve.
3. Prepare 1000 ppm K by dissolving 1.9084 g AR grade KCl (dry) at 60°C for 1 hour. Dissolve in distilled water and make up the volume to 1 litre.
4. Dilute required portions of this solution with ammonium acetate to give 10 to 40 ppm of K.
5. Attach appropriate filter, adjust gas and air pressure, set the flame photometer readings at zero for the blank (ammonium acetate) and 100 for 40 ppm K.
6. Plot a graph with the readings of the flame photometer against concentration of K (10,15,20,25,30,35 ppm) and find out K in unknown sample.
Calculation:
where R= ppm of K in extract from standard curve.