The following article will guide you to know how to Isolate and Purify the Recombinant Proteins from the Transformed Host Cells.
Extraction of Protein from Selected Transformed Cells:
If the protein is secreted out then we don’t have to go for this step. In this case the fermentation medium is filtered to separate the host cells and then subjected to purification. If the protein is expressed in the cytoplasm, plasma membrane or in any organelle then we have to first obtain the cell the culture medium where they are grown. This is called as cell recovery and can be done either by centrifugation or filtration.
Then we break open the cells either by disrupting it by using suitable buffer system. This crude homogenate thus obtained is then subjected to density gradient centrifugation. The cell debris get settled down as pellet whereas proteins along with lipids, nucleic acids and carbohydrates remains in the supernatant. All these unnecessary macromolecules are then removed one by one to get a protein mixture.
The lipids can be removed by passing the solution through a glass wool or a cloth and nucleic acids can be removed by treating with nuclease or by precipitation followed by centrifugation. Before we go for the purification of extracted protein it is necessary to obtain a good quantity of it.
This is done by precipitating the protein either by salting out or by organic solvents (e.g., acetone) or by changing the pH (isoelectric precipitation).
Purification of Extracted Protein Sample:
The protein precipitate thus obtained still has many impurities like ions and smaller molecules, oligosaccharides, etc.
To remove all the impurities we may follow any one of the following techniques:
(a) Dialysis:
The solution of impure protein is enclosed in a partially permeable membrane. Smaller solute molecules and ions pass through this, while the larger protein molecules are held back.
(b) Gel-Filtration Chromatography:
This is also called size-exclusion chromatography. In this technique the solution of impure protein is allowed to pass through a column packed with beads of a polymer. The large protein molecules can move freely around the beads and pass through quickly. Smaller molecules and ions have to pass through the polymer network inside the beads and so they take longer to travel through the column.
(c) Ion-Exchange Chromatography:
The solution of impure protein passes through a column packed with beads of a polymer that have charged groups attached. If the beads have negative charges, then the protein molecules with an overall positive charge stick to them, while others wash through. The molecules can be released from the column by changing the pH or salt concentration of the solution washing through.
(d) Affinity Chromatography:
In this technique we add amino acid tags to the protein that give it a new property, which is to bind very tightly to a particular substance. This binding can then be used to isolate the protein away from all the other materials in the cell which do not bind this substance. A common example is the use of “his-tags” to help purify a protein.
A his-tag is a string of (usually six) histidine residues which is added to the N or C terminus by adding the nucleotides encoding for these extra ‘his’ residues when the gene is cloned. The presence of the extra his-tag on the protein makes it bind very tightly to metal ions such as Ni2+.
The resin containing Ni2+ ions is placed in a column, and a crude extract of E. coli cells that contain the his-tagged protein is passed down the column. The E. coli proteins will pass through the column but the protein with the attached his-tag will be bound to the column.
If a buffer containing a salt such as imidazole is subsequently passed down the column, the bound his-tagged protein is now released from the nickel ions, and can be collected in the buffer as it flows out of the column.
Identification of Recombinant Protein from a Mixture of Purified Proteins:
When the protein sample is purified it contains a mixture of various proteins. We have to identify and isolate our recombinant protein from that mixture.
Following techniques are regularly followed for the identification of a specific protein molecule in a mixture of polypeptides:
(a) Western Blotting:
This technique is also called as immuno-blotting.
It is a powerful method for detecting a particular protein in a complex mixture.
This involves following four steps;
Step 1:
After a protein mixture has been electrophoresed through an SDS gel, the separated bands are transferred (blotted) from the gel onto a porous membrane.
Step 2:
The membrane is flooded with a solution of antibody (Ab1) specific for the desired protein. Only the band containing this protein binds the antibody, forming a layer of antibody molecules (although their position cannot be seen at this point). After sufficient time for binding, the membrane is washed to remove unbound Ab1.
Step 3:
The membrane is incubated with a second antibody (Ab2) that binds to the bound Ab1. This second antibody is covalently linked to alkaline phosphatase, which catalyses a chromogenic reaction.
Step 4:
Finally, the substrate is added and a deep purple precipitate forms, marking the band containing the desired protein.
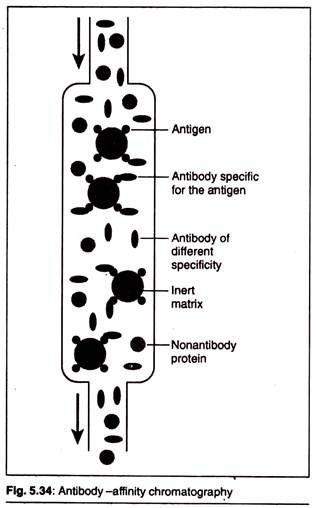
(b) Antibody-Affinity Chromatography:
In antibody-affinity chromatography, a specific antibody, developed against our recombinant protein, is covalently attached to beads packed in a chromatography column. Only recombinant protein with high affinity for the antibody is retained by the column; all the non-binding proteins flow through. The bound recombinant protein is eluted with an acidic solution, which disrupts the antigen-antibody complexes.