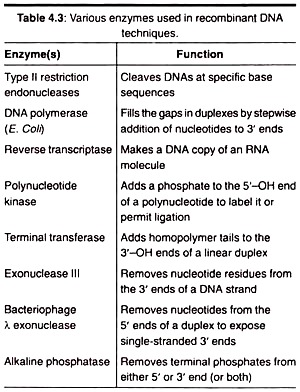
The following points highlight the eight main enzymes used for generation of a recombinant DNA. The enzymes are: 1. Restriction Endonucleases 2. Alkaline Phosphatases 3. Reverse Transcriptase 4. Polynucleotide Kinase 5. DNA Ligase 6. Nucleases 7. Terminal Deoxynucleotide Transferases (TDNT) 8. DNA Polymerase.
Enzyme Type # 1.
Restriction Endonucleases:
A commonly used tool in molecular biology is restriction endonucleases. Restriction endonucleases, otherwise known as restriction enzymes, are molecular scissors that can cut double-stranded DNA at a specific base-pair sequence.
Each type of restriction enzyme recognizes a characteristic sequence of nucleotides that is known as its recognition site. Researchers can use these enzymes to cut DNA in a predictable and precise manner.
Discovery of Restriction Endonucleases:
Scientific discoveries often have their origin in seemingly unimportant observation that receive little attention by researchers before their general significance is appreciated. In case of genetic engineering, the original observation was that bacteria use enzymes to defend themselves against viruses.
Most organisms eventually evolve means of defending themselves from predators and parasites, and bacteria are no exception. Among the natural enemies of bacteria are bacteriophages, viruses that infect bacteria and multiply within them.
At some point, they cause the bacterial cells to burst, releasing thousands more viruses. Through natural selection, some types of bacteria have acquired powerful weapons against these viruses; they contain enzymes called restriction endonucleases that fragment the viral DNA as soon as it enters the bacterial cell.
Many restriction endonucleases recognize specific nucleotide sequences in a DNA strand, bind to the DNA at those sequences, and cleave the DNA at a particular place within the recognition sequence. Why don’t restriction endonucleases cleave the bacterial cells’ own DNA as well as that of the viruses?
The answer to this question is that bacteria modify their own DNA, using other enzymes known as methylases to add methyl (—CH3) groups to some of the nucleotides in the bacterial DNA. When nucleotides within a restriction endonuclease’s recognition sequence have been methylated, the endonuclease cannot bind to that sequence.
Consequently, the bacterial DNA is protected from being degraded at that site. Viral DNA, on the other hand, has not been methylated and, therefore, is not protected from enzymatic cleavage.
Types of Restriction Endonucleases:
All restriction enzymes fall into one of three classes, basing upon their molecular structure and need for specific co-factors.
I. Class I Endonucleases:
These have a molecular weight around 300,000 Daltons, Eire composed of non-identical subunits, and require Mg2+, ATP (adenosine triphosphate), and SAM (S-adenosylmethionine) as cofactors for activity. Not used in RDT experiments.
II. Class II Endonucleases:
These are much smaller, with molecular weights in the range of 20,000 to 100,000 Daltons. They have identical sub-units and require only Mg2+ as a cofactor (Nathans and Smith, 1975). Only class II endonucleases are used in RDT experiments due to their site specific cleavage action.
III. Class III Endonucleases:
These are large molecules, with a molecular weight of around 200,000 Daltons, composed of non- identical sub-units. These enzymes differ from enzymes of other two classes in that they require both Mg2+ and ATP but not SAM as co-factors. Class III endonucleases are the rarest of three types. Not used in RDT experiments.
Nomenclature:
As a large number of restriction enzymes have been discovered, a uniform nomenclature system is adopted to avoid confusion.
This nomenclature was first proposed by Smith and Nattens in 1973.
1. The first letter of restriction enzymes (RE) should be from first letter of the species name of organism from which the enzyme is isolated.
The letter should be written in capitals and italics, e.g., RE from E. coli will have E as starting letter.
2. The second and third letters of RE should be from the first and second letters of genus name of the organism. The letter should be written in lower case and should be in italics, e.g., RE from E coli will have Eco as starting words.
3. If the RE is isolated from particular strain of an organism, then that should be written as fourth letter. It should be in capitals and not in italics. For example, RE from E. coli R strain will be written as Eco R.
4. If the RE isolated is the first of its kind from that particular organism, then the number I should be given. If already two REs are isolated, then number III should be given for new restriction enzymes. The number should be written in roman, e.g., the first E. coli RE should be written as Eco RI whereas the third restriction enzyme isolated from E. coli R strains should be written as Eco RIII.
Recognition Sequences:
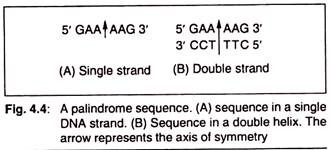
The recognition sequences for class II endonucleases form palindromes with rotational symmetry. In a palindrome, the base sequence in the second half of a DNA strand is the mirror image of sequence in its first half (Fig. 4.2). But in a palindrome with rotational symmetry, the base sequence in the first half of one strand of a DNA double helix is the mirror image of second half of its complementary strand (Fig 4.3).
Thus in such palindromes, the base sequence in both the strands of a DNA duplex reads the same when read from the same end (either 5′ or 3′) of both the strands.
Mechanism of Action of Restriction Endonucleases:
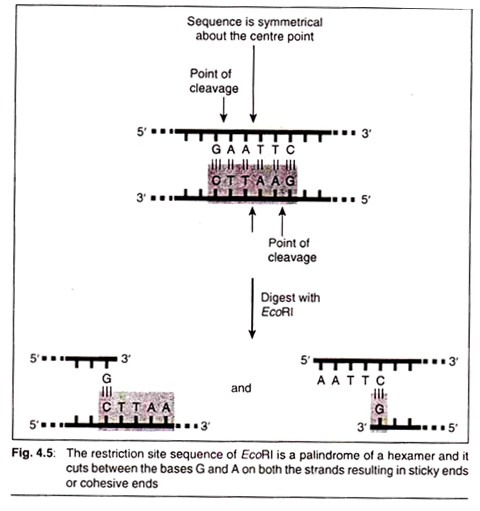
EcoRI can be taken as an example of class II endonucleases and its works can be seen. When the restriction endonuclease encounters its respective restriction site sequence (5′ GAATTC 3′), it cleaves each backbone between the G and the closest A base residues.
Once the cuts have been made, the resulting fragments are held together only by relatively weak hydrogen bonds that hold the complementary bases to each other. The weakness of these bonds allows the DNA fragments to separate from each other. Each resulting fragment has a protruding 5′ end composed of unpaired bases (Fig. 4.4).
Other enzymes also create cuts in the DNA backbone in the same manner, which results in protruding 3′ ends. Protruding ends—both 3′ and 5’—are sometimes called ‘sticky ends’ because they tend to bond with complementary sequences of bases.
In other words, if an unpaired length of bases (5′ A A T T 3′) encounters another unpaired length with the sequence (3′ T T A A 5′) they will bond to each other—they are ‘sticky’ for each other. Ligase enzymes are then used to join the phosphate backbones of two molecules.
The cellular origin, or even the species origin, of the sticky ends does not affect their stickiness. Any pair of complementary sequences will tend to bond, even if one of the sequences comes from a length of human DNA, and the other comes from a length of bacterial DNA.
In fact, it is this quality of stickiness that allows the production of recombinant DNA molecules (molecules which are composed of DNA from different sources and have given birth to a powerful technology and industry) the genetic engineering or the recombinant DNA.
Examples of some other restriction enzymes, their mode of cutting and generation of 5′ overhangs and 3′ overhangs, are illustrated in (Fig. 4.5). Sticky ends (also called cohesive ends or overhanging heads) are useful for DNA cloning because complementary sequences anneal and can be ligated directly by DNA ligase.
If two different DNA samples cleaved with the same type of restriction enzymes are mixed together in the presence of DNA ligase, a recombinant DNA molecule can be generated. This is possible because of the presence of same type of sticky ends.
The complementary sequences of sticky ends from the unrelated DNA samples will anneal together and are finally joined by the DNA ligase enzyme to form the recombinant DNA molecule.
Some restriction enzymes, on the other hand, cut both the strands of a DNA molecule at the same site so that the resulting termini or ends have blunt or flush ends (Fig. 4.6) in which the two strands end at the same point. The blunt ends also can effectively be utilized as recombinants followed by some end point modifications.
As the list of restriction enzymes grew and their recognition sequences were identified, it was found in some cases that more than one enzyme could recognize the same sequence. RJ Roberts conferred the term isoschizomer (same cutter) on restriction enzymes that recognized the same DNA sequence.
Star Activity:
Sometimes restriction enzymes recognize and cleave the DNA strand at the recognition site with asymmetrical palindromic sequence; for example, Bam HI cuts at the sequence GA TCC, but under extreme conditions, as in low ionic strength it will cleave in any of the following sequences NGA TCC, GPOA TCC, GGNTCC. Such an activity of the restriction endonucleases is called star activity.
Difficulties Associated with Restriction Digestion:
There are certain limitations for restriction endonucleases.
Those are as follows:
1. Different enzymes can generate the same ends. For example, Sau3A1 and BamHI produce GATC−. If these two enzymes are used then they will form the same ends which will not ligate later.
2. Restriction endonuclease preparation should be free from nucleases, otherwise the ends produced by theses enzymes can be degraded by exonucleases.
3. Most restriction endonucleases are very stable when stored at -20°C in the recommended storage buffer. Exposure to temperature above -20°C can decrease the activity of these enzymes.
4. The specificity of some restriction endonuclease is affected by the type of buffer used.
5. Secondary structures in DNA often interfere with recognition or cleavage by endonuclease.
Works of Reference for Restriction Digestion:
Nowadays we get a complete online database for every available restriction endonuclease. REBASE provides a current review of restriction enzymes, whether and where they can be obtained, a list of publications concerning the enzymes, and much more. At this site, you can search DNA sequences for their open reading frames and cleavage sites can be searched out.
Uses of Restriction Endonucleases:
Restriction enzymes have been used for sequence analysis, cloning and amplifying DNA. DNA from animal viruses bacteriophages contains 5,000 to 50,000 base pairs. It is important to know the primary structure of DNA, i.e., the sequence of bases, for decoding the information stored in genes, for understanding gene structure and regulation at molecular level.
The discovery of restriction enzymes was a major breakthrough in sequence analysis of DNA. By using combinations of different restriction enzymes it is possible to hydrolyse large DNA molecules into fragments less than 300 base pairs in length.
These fragments can then be used for sequence analysis and are arranged into a physical map of the chromosome. This is a slow and laborious process. The mapping of entire 5,000 base pair DNA of the virus SV40 into some 100 fragments has taken several years. Complete sequence analysis of the fragments would take much longer.
The DNA fragments produced by restriction endonucleases can covalently be linked in vitro to linear plasmid DNA or to lambda phage DNA. The recombinant DNA species produced can be inserted into E. coli by transformation.
Each transformed cell can then be grown as a separate clone. By this method a complex genome can be broken down into thousands or millions of pieces, and each piece is isolated to form a separate clone.
Enzyme Type # 2.
Alkaline Phosphatases:
Alkaline phosphatase is a glycoprotein with two identical subunits. The cohesive ends of broken plasmids, instead of joining with foreign DNA, join the cohesive end of the same DNA molecules and get re-circularized. To overcome this problem the restricted plasmid is treated with an enzyme, alkaline phosphatase, that digests the terminal phosphoryl group.
The restriction fragments of the foreign DNA to be cloned are not treated with alkaline phosphatase.
Therefore, the 5′ end of foreign DNA fragment can covalently join to 3′ end of the plasmid. The recombinant DNA thus obtained has a nick with 3′ and 5′ P hydroxy ends. Ligase will only join 3′ and 5′ ends of recombinant DNA together if the 5′ end is phosphorylated.
Thus, alkaline phosphatase and ligase prevent re-circularization of the vector and increase the frequency of production of recombinant DNA molecules. The nicks between two 3′ ends fragment and vector DNA are repaired inside the bacterial cells during the transformation.
Enzyme Type # 3.
Reverse Transcriptase:
Many times we do not get our gene of interest rather its mRNA. In this case reverse transcriptase enzyme can be used to prepare a double stranded DNA (our gene of interest) from the available single-stranded mRNA (template) by a process called reverse transcription.
Reverse transcriptase enzyme is also called RNA dependent DNA polymerase. These enzymes are present in most of the RNA tumour viruses and retroviruses.
Enzyme Type # 4.
Polynucleotide Kinase:
Kinase is the group of enzyme, which adds a free pyrophosphate (PO4) to a wide variety of substrates like proteins, DNA and RNA. It uses ATP as cofactor and adds a phosphate by breaking the ATP into ADP and pyrophosphate. It is widely used in molecular biology and genetic engineering to add radio-labelled phosphates. In RDT experiments mostly T4 polynucleotide kinase is used.
Enzyme Type # 5.
DNA Ligase:
Recombinant DNA experiments require the joining of two different DNA segments or fragments in vitro. The ends generated by some RE will be either cohesive (sticky) or blunt. The cohesive ends will anneal (join) themselves by forming hydrogen bonds. But the segments annealed thus are weak and do not withstand experimental conditions.
To get a stable joining, the DNA should be joined by using an enzyme called ligase. In the case of blunt ends we use linker or adaptors for successful ligation.
There are two types of DNA ligases:
(a) T4 DNA Ligase:
Naturally coded by T4 bacteriophage. The catalytic activity of the enzyme requires the presence of ATP as cofactor and Mg++. This is predominantly used in RDT experiments.
(b) NAD+ dependent DNA Ligase:
Naturally found in E. coli. Uses NAD+ as a co-factor and only found in bacteria.
Mechanism of Action:
The cofactor is first spited (ATP→ AMP + 2Pi) and then AMP binds to the enzyme to form the enzyme-AMP complex. This complex then binds to the nick or break (with 5′ −PO4 and 3′ −OH) and makes a covalent bond in the phosphodiester chain. The ligase reaction is carried out at 4°C for better results.
Enzyme Type # 6.
Nucleases:
Nucleases are group of enzymes which cleave or cut the genetic material (DNA or RNA). These enzymes are further classified into two types based upon the substrate on which they act. Nucleases which act on or cut the DNA are classified as DNases, whereas those which act on the RNA are called RNases.
DNases are further classified into two types based upon the position where they act. DNases that act on the ends or terminal regions of DNA are called exonucleases and those that act at a nonspecific region in the centre of the DNA are called endonucleases.
Exonucleases require a DNA strand with at least two 5′ and 3′ ends. They cannot act on DNA which is circular. Endonucleases can act on circular DNA and do not require any free DNA ends (i.e., 5 or 3 end). Exonucleases release nucleotides, whereas endonucleases release short segments of DNA. The frequently used nucleases in the experiments of RDT are Exonuclease III and bacteriophage exonuclease.
Enzyme Type # 7.
Terminal Deoxynucleotide Transferases (TDNT):
This is a polymerase which adds nucleotides at 3′-OH end (like Klenow fragment) but does not require any complementary sequence and does not copy any DNA sequence (unlike Klenow fragment). Terminal deoxynucleotide transferase (TDNT) adds nucleotide whatever comes into its active site and it does not show any preference for any nucleotide.
Enzyme Type # 8.
DNA Polymerase:
These are mostly used when we are carrying out the cloning of the recombinant DNA in the prokaryotic host cells like E. coli. Then we fill the gaps in duplexes by stepwise addition of nucleotide to 3′ ends.