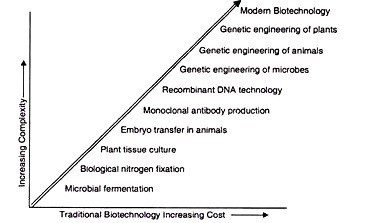
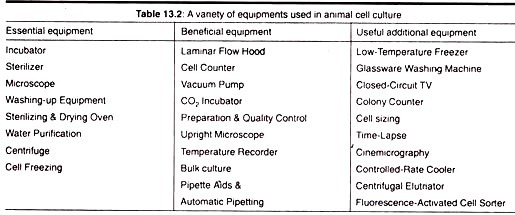
In this article we will discuss about:- 1. Meaning of Plant Tissue Culture 2. Culture Media 3. Applications of Tissue Culture 4. Callus Culture 5. Organogenesis 6. Embryogenesis 7. Culture of Zygotic Embryo 8. Microspore Culture 9. Isolation of Plant Protoplast 10. Protoplast Fusion 11. Cybrids 12. Meristem Culture 13. Agrobacterium Mediated Transformation System and Other Details.
Meaning of Plant Tissue Culture:
In tissue culture, when a piece of plant is placed in a test tube (in vitro) under sterile conditions and provided with nutrients, it will produce tiny replicas of its parent. This is mainly due to the plasticity’ and totipotency’ exhibited by plants. Plasticity allows the plant to alter their metabolism, growth and development, to best suit their environment.
When tissues are cultured in vitro, their plasticity allows one type of tissue or organ to be initiated from another type and thus regeneration of whole plants occur. This depends on the concept that all plant cells can, if given the correct stimuli, express the total genetic potential which is known as totipotency.
The plants, when multiplied by tissue culture or by more conventional methods, distinct from that of being grown from seeds, all the offspring’s from a single plant are members of a group called a “clone”, meaning that their genetic make-up is identical to each other and to their lone parent. Somaclonal variations are variations displayed among plants derived from any form of cell culture.
Tissue culture serves a number of purposes like mass production of plants, establishing or maintaining ‘virus free’ stock etc. The natural capacity of plants to reproduce vegetatively or asexually is the basis of tissue culture in vitro (in glass).
In vitro cultures of plant parts provide an ideal tool for research in studying a wide range of aspects of plant science like e.g. primary and secondary metabolism, morphogenesis, cyto-differentiation, physiology, production of hybrids etc. Tissue cultured plants are used for commercial propagation. Haploids from pollen grains are important in plant breeding and genetic studies.
Plant tissue culture begins with the initiation of callus cultures (= dedifferentiated masses of rapidly dividing cell cultures on semisolid, agar based nutrient media) which are generated by exposing sterile pieces of a plant, to plant growth regulators.
In intact plants, cell division is restricted to stem and root tip meristems regulated by the natural hormones present in the cells. In plant tissue culture, the added plant growth regulators bind to receptors in the plant cells causing all the cells to resume division and growth.
Culture Media:
The significant factor in the success of the technology of cell, tissue or organ culture is the choice of nutritional components and growth regulators. There are different media and growth hormones that are used, with reproducible results in culturing plant cells, protoplasts, anthers or meristems.
Most of the plant cell lines can be cultured, in completely defined media, although some of them need complex ingredients like coconut milk. However, the choice of a medium is dictated by the purpose of the culture, the species or type of plant and its developmental stage.
Composition:
i. Macronutrients:
Sources of nitrogen, potassium, phosphorus, sulphur and calcium are required for healthy plant growth. Sodium and potassium ions can be tolerated upto 50-60 nM. Nitrogen is provided as nitrate, though some media contain both ammonia and nitrate source of nitrogen.
The pH of the medium drops when ammonium source is used and rise when nitrate source is used. Though some species use ammonium or glutamine, high concentrations of these can be toxic due to shifts in pH. Phosphates provide phosphorus and also act as buffer.
ii. Micronutrients:
Several trace elements are required for the growth of plant cells, tissues and organs. Iodide, though not essential, stimulates cell growth. Iron is added in a chelated form either as an EDTA salt or as agricultural chelates e.g. Sequestrene 330 Fe.
iii. Carbon sources:
Since most cultures cannot photosynthesise enough sugars, for growth, a carbon source is supplied. This in the form of sucrose in the range of 20-30 g/litre is added to the medium. Glucose is used less often and fructose only occasionally.
In most of the cultures, sucrose is readily taken up in one or two days and converted to starch granules within the cells. Some media contain m-inositol which is a beneficial component for cell wall metabolism.
iv. Vitamins:
Intact soil grown plants synthesise all vitamins required for their growth. In vitro cultures require thiamine. Pyridoxine, nicotinic acid and myoinositol have enhancing effects on growth and development. All these vitamins are stable during autoclaving. Other vitamins like biotin, pantothenic acid, folic acid, choline chloride, p-aminobenzoic acid, riboflavin, vitamin B12 and ascorbic acid are added at 1mg/Iitre or less in some instances, especially when cells or protoplasts are cultured.
v. Plant growth regulators:
These include both naturally occurring plant hormones (phytohormones) and synthetic ones. Most of the synthetic ones are structural analogs of the natural hormones and bind to the same receptors.
Plant growth regulators used in plant tissue culture are auxins, cytokinins, gibberellins and abscisic acid. In most of the cultures only auxins and cytokinins are used.
a. Auxins:
Auxins cause cell elongation, apical dominance and adventitious root formation. In plant tissue culture, auxins are used either alone or with Cytokinins or other categories of plant growth regulators (PGR) for callus induction, organogenesis and somatic embryogenesis.
Both natural and synthetic auxins are used in tissue culture:
i. Natural ones:
Indole-3-acetic acid (IAA), Indole-3-butyric acid (IBA)
ii. Synthetic ones:
1-Naphthalene acetic acid (NAA),
2,4-Dichloro phenoxy acetic acid (2,4-D)
Auxins are used in the range of 1-5 µM. IAA is unstable in solution and is easily oxidised and conjugated to inactive forms by plant cells. IBA is more stable. IAA and IBA are not heat stable. Hence they cannot be co- autoclaved with the medium. They are incorporated in the medium in sterile form. 2,4-D is more potent than NAA, followed by IBA and IAA. 2,4-D and NAA can be autoclaved. Media with IAA and IBA must be used immediately.
b. Cytokinins:
Cytokinins promote cell division and are used for initiation of culture or for long term growth. They are used for initiation of shoots from calli either along with low concentrations of auxin or without auxins. The more commonly used cytokinin is kinetin, since it induces development and multiplication of shoot bud. Zeatin was the first naturally occurring cytokinin to be identified. Zeatin is a purine derivative.
The other more commonly used cytokinins are the synthetic kinetin (6-furfural laminopurine) and 6-benzyl aminopurine (BAP). BAP is also known as Ng benzyl adenine (BA). Cytokinin like activity is exhibited by adenine and adenosine, particularly at higher concentrations. Most of the cytokinins except zeatin and its derivatives can be autoclaved with the media and are used in the range of 1-50 µm.
c. Gibberellins:
Gibberellins were first obtained from the “foolish seeding blight” of rice caused by Gibberella fujikuori. Gibberellins cause stem elongation, flowering, breaking of dormancy of seeds, parthenocarpic development of fruits etc. in whole plants. In plants there are more than fifty different compounds classified as gibberellins. However, only GA3, gibberellic acid and GA417 are used in plant tissue culture. Gibberellins, in plant tissue culture inhibit induction of organogenesis, especially adventitious root formation.
d. Abscisic acid (ABA):
ABA regulates closure of stomata and cause bud and seed dormancy. In plant tissue culture, ABA promotes maturation and germination of normal somatic embryos.
Ethylene:
Ethylene gas is the main plant growth regulator (PGR), responsible for abscission and flower senescence. Its role in plant tissue culture is not known.
Organic additives:
These do not promote growth but act as nutrients. Complex ingradients like coconut milk, yeast extract, fruit juices and casein hydrolysates are used. They provide amino acids, vitamins, growth regulators etc. which can enhance plant cell growth.
Mannitol, sorbitol or combinations of these are used as an osmoticum (increase osmolarity). A concentration of 100 g/litre (0.6M) is generally used.
Organic acids like citric acid, fumaric acid, malic acid or succinic acid are used to maintain pH.
N.B.: Auxins (IAA, NAA, IBA and 2,4-D) should be dissolved in minimum amount 0.1N NaOH or ethyl alcohol and final volume made upto one ml.
Cytokinins (BAP and Kinetin) should be dissolved in minimum amount of either 0-1N NaOH or ethyl alcohol and final volume made upto one ml.
Most of the cytokinins except Zeatin and its derivatives can be autoclaved along with media.
IAA and IBA should be filter sterilised and incorporated into autoclaved media.
2,4-D and NAA can be added to media and autocalved.
vi. Gelling agent:
Both liquid and semisolid media can be used depending on the culture. Most of the media used in plant tissue culture need semi-solid or gelled media. Agar-agar obtained from the sea weed is the most commonly used gelling agent.
Applications of Tissue Culture:
1. Micropropagation.
2. Somatic embryogenesis.
3. Virus elimination.
4. Embryo rescue.
5. Haploid production.
6. Ploidy manipulation.
7. Germ plasm storage and exchange.
8. Secondary production of metabolites.
9. Modification by somaclonal variation.
10. Plant gene transfer by protoplast fusion.
11. Gene introduction by Agrobacterium or other means.
Callus Culture:
Callus is the initiation and proliferation of undifferentiated parenchyma cells from an explant on a chemically defined medium in vitro.
Almost any tissue can be used as an ex- plant. However, when an explant comes from relatively young region, it will have a greater potential for cell division as well as regeneration. The explant should be small enough in order that it will be of fairly homogeneous cells, but at the same time should be capable of sustained cell division.
The medium should have a mixture of salts, hormones, suitable pH and agar for gelling. Callus cultures are incubated in dark. The explants generally used are shoots, buds, roots and embryos. These should be surface sterilized before inoculation.
Principle:
When an explant is inoculated on a nutrient medium supplemented with growth regulators and incubated, undifferentiated masses of tissue (callus) develop.
Requirements:
Nutrient medium:
Murashige and Skoog’s (1962) medium supplemented with growth regulators.
1. Explant—seeds of Phaseolus.
2. Ethanol.
3. 1 in 1000 HgCl2/sodium hypochlorite solution.
4. Sterile distilled water.
5. Sterile Petri plates with moist filter paper.
6. 45% acetic acid.
7. Acetocarmine.
8. Slides and coverslips.
9. Inoculating needles.
10. Scalpel.
11. Long forceps.
12. Dark culture room at 25°C.
13. Laminar clean air flow hood.
Procedure:
1. Sterilize healthy Phaseolus seeds with unbroken testa, by first washing in water and dipping them in 95% ethanol for 30 seconds.
2. Transfer them to 1 in 1000 HgCl2 or sodium hypochlorite solution and keep for 5-10 minutes shaking occasionally.
3. Rinse thoroughly with sterile distilled water, 5-6 times.
4. Place them in sterile Petri plates with moist filter paper and incubate at room temperature in dark for 2-5 days.
5. Cut hypocotyl, shoot tips or leaf segments measuring 5.0-7.0 mm using a sterile scalpel and use as explants to inoculate on nutrient medium. (composition given bellow).
6. Incubate at 25°C in dark. Callus start developing within 5—8 days.
7. Make histological studies by squashing them in 45% acetic acid, stain by acetocarmic. Use the remaining callus for further studies by sub-culturing them on the same medium in tubes.
Method for Preparation:
1. Dissolve compounds 1-10 in ascending numerical order in 300 ml double distilled water.
2. To this add 1 ml each of solutions 13, 14, 15 and make the volume upto’400ml. – stock solution A.
3. Dissolve 11 and 15 in 50 ml double distilled water, make the volume upto 100 ml. – stock solution B.
4. Dissolve 30 g sucrose in 600 ml double distilled water. Add to this 80 ml of solution A and 2 ml of solution B. Stir well and dilute to 970 ml.
5. To this 970 ml add 0.2 ml each of solutions 16, 17, 18 and 19, adjust pH to 5.5 with 0.1 M NaOH and make up the volume to 1000 ml.
6. Add selected plant growth regulators (stock solutions of plant growth regulators 1 mg/ml should be prepared and stored at 4°C).
7. NAA, IAA and 2, 4-D to be dissolved in 0.1 N NaOH or ethyl alcohol.
8. Kinetin Zeatin and BAP can be dissolved in dilute NaOH or a drop of ethyl alcohol.
For callus initiation:
are some of the combinations used. Add 8.0 g/l agar: (either premelt agar or after adding agar to the medium keep on a water bath), stir well and distribute 5 ml of medium each in 6×1 inch tubes or in Erlenmeyer flasks (30 ml medium in 100 ml flasks), plug with non-absorbent cotton, cover the plugs from top and autoclave at 121°C and 15-16 lb pressure for 15 minutes.
Inoculate explants on cooled media aseptically.
Incubate in dark at 25°C.
Organogenesis:
Organogenesis is the de novo adventitious development of non-autonomous organs. It occurs directly from the explant (direct organogenesis) or indirectly through calli (indirect organogenesis). Indirect organogenesis, when a shoot as explant is placed on a medium with more cytokinin and less auxin, multiple shoot formation occurs.
Indirect organogenesis occurs when the medium is supplemented with more auxin and less cytokinin leading to callus formation and root development. Depending on the explant 2, 4-D at a concentration of 50 mg/l will develop callus. For development of roots, IBA at 0.1 mg/l is used more commonly. The external application of growth regulators (auxins and cytokinins) disturbs its original concentration in the explant, leading to shoot or root and callus development.
The ability of the cells of calli to differentiate into roots and shoots is known as organogenesis. Each cell is totipotent and is capable of developing into an entire plant, when they are supplied with correct nutrients and environment. The process is not dependent on pre-existing initials of roots or shoots.
One or a few of the cells of a callus give rise to roots and shoots. These cells become activated and undergo a series of divisions which lead to the development of a meristemoid. This meristemoid is an aggregation of meristem like cells which give rise to either a root or a shoot primordium.
The manipulation of the ratio of growth regulator is an important factor in organogenesis. Generally high ratio of cytokinin to auxin results in shoot formation and a high ratio of auxin to cytokinin leads to development of roots.
Requirements:
1. Culture medium:
M.S. medium supplemented with growth regulators to raise callus form.
2. Explant:
Pelargonium tomentosum or plant sprouts of ginger or turmeric.
3. Sub-cultured callus on fresh medium of the growth regulators and their combinations:
4. Culture vessels.
5. Wide mouthed and autoclaved 100 ml Erlenmeyer flasks used for standard tissue culture with MS medium plus growth regulators as mentioned above.
6. Scalpel, inoculating needles, long forceps etc.
7. Disinfectant—1 in 1000 HgCl2 or Na hypochlorite and sterile distilled water.
Procedure:
1. Inoculate explants and grow callus.
2. Subculture calli to freshly prepared media supplemented with the above cited combinations of growth regulators.
3. Incubate cultures in temperature controlled rooms with fluorescent lamps.
4. Observe for organogenesis (root and shoot) Medium with 0.05 mg/l BAP and 0.05 mg/l NAA gives better organogenesis of Pelargonium tomentosum.
5. Isolate these from callus material and either maintain them or harden them.
Embryogenesis:
Embryos can, not only be produced from fertilized eggs, but can also be produced from somatic tissues that are differentiated by first subjecting it to the action of an auxin and then transferred to a medium without auxin. Here it appears that the auxin rejuvenates the cell to such an extent that it reacquires the ability of a zygote to produce an embryo. Such embryos are known as somatic embryos.
These are diploid embryos, in contrast to the haploid embryos that arise from pollen grains. Somatic embryos do not have vascular connection with the mother tissue and hence can easily be separated. The first leaves of such embryos will have cotyledonary characters.
In somatic embryogenensis, as in zygotic embryogenesis, the embryoid passes through globular, heart shaped and torpedo shaped stages.
Two types of induction of embryogenesis are there:
1. Pre-embryogenic determined cells (PEDC).
2. Induced embryogenic determined cells (IEDC).
An embryo has globular, heart shaped and torpedo shaped stages, after which desiccation should occur for it to differentiate. This desiccation does not take place in in vitro cultures and hence it has to be induced in order that they develop into normal embryos.
Desiccation can be induced in vitro by:
(i) ABA
(ii) Polyethylene glycol (PEG)
(iii) High concentration of sucrose (reverse osmosis)
(iv) Keeping it exposed in laminar clean air flow.
When callus develops, it may be white, friable, shining or compact light brown coloured one. The former one (friable callus) will produce embryos and the latter (light brown) may develop into friable callus at certain points, from where embryos may be produced.
An immature zygotic embryo when placed on a medium, will germinate and give rise to a plant or give rise to many plants-repetitive embryogenesis.
Requirements:
1. Donor plant: Dacus carota (carrot).
2. Culture medium: M.S. medium with and without auxin.
Few chemical factors have considerable effect on embryogenesis:
(i) A substantial amount of reduced nitrogen (NH+4) is needed,
(ii) Medium should be supplemented with amino acids like glutamine,
(iii) K+ is the one that is effective among other ions and
(iv) Auxin should be added at 0.5 or 1.0 mg/l of concentration.
3. Culture room (temperature 25°C) with 1000 lux fluorescent lamps.
4. Scalpel, needles, forceps etc.
5. Disinfectant, one in 1000 HgCl2/Na hypochlorite and sterile distilled water.
6. Filter paper caps or strips.
Procedure:
There are two successive cultures for embryo- genesis:
(i) Primary tissue (explant) grown on a medium with auxin where there is no differentiation or embryogenic induction,
(ii) Culture of this undifferentiated tissue in an auxin free medium where embryogenesis occurs.
(i) Primary cultures:
1. Sterilize pieces of explant in Na hypochlorite solution for 10-15 minutes and wash 5-6 times with sterile distilled water.
2. Trim the ends of this piece of stem with sterile scalpel and cut central piece into bits.
3. Place these pieces vertically or horizontally in tubes with medium having auxin and incubate in dark.
4. Nodule like proembryonic masses arise. This grows slowly and hence should be transferred after1 ½ month.
(ii) Secondary culture:
1. Use the same medium without auxin and glutamine to which nodules of primary culture (callus with nodular growth) are transferred and incubated in light.
Nodules grow into embryos after a few weeks. Embryos will be small heart shaped ones and there will be intertwining of these embryos.
2. Transfer mature ones to filter paper bridges dipped in liquid M.S. medium at 1/2 concentration with 5 g/l sucrose and incubate in growth chamber.
3. When roots develop, transfer to pots with soil mixture.
Culture of Zygotic Embryo:
When wide crosses are made between two parental plants, the resulting diploid zygote may abort and the triploid endosperm may not develop fully. In such cases, the embryos are or can be rescued in vitro.
Embryo cultures are made to observe morphogenesis from isolated embryos. Its application is to produce hybrids.
Requirements:
1. M.S. medium:
Modified with 120 g/l sucrose, 400 mg/l glutamine, 1 mg/l Vitamin B, lmg/l vitamin B2, 7 g/l Agar.
2. Fruits of Capsella bursa-pastorisl Carica papaya.
3. Scalpel, inoculating needles etc.
4. Culture room at 25°C with continuous light provided by 1000 lux fluroscent tubes.
5. Disinfectant:
1/1000 HgCl2/Na hypochlorite, Distilled water.
Procedure:
1. Surface sterilise the seeds using HgCI2 solution (5-10 minutes) wash several times with sterile distilled water.
2. Dissect the fruits in a solution with 120 g/l sucrose and 1.75 g/l soft agar in sterile Petriplates to immobilise ovules and prevent dessication. (This dissecting medium should first be tyndallised since sterilization temperature affects survival and growth of embryos. For this, heat the medium upto 100°C twice with an interval of 24 hours. First heating exposure to 100°C should be for 5 minutes and the second for 15 minutes.)
Pour the medium, allow to cool and on a sterile slide or Petri plate cut open the surface sterilized fruits with a sterile scalpel and wash the seeds with placenta in a few drops of dissecting medium.
3. Detach seed from placenta to an adjacent drop of dissecting medium.
Make.an incision with a sterile microscalpel opposite to the micropyle and press the micropyle so that embryo gets ejected.
4. Remove the endosperm attached to the embryo and by a micropipette remove embryo and place it in an engraved (marking made on the lower side of the lower half of the Petri dish as 1 cm squares) Petri plate with medium.
5. Seal the Petri plate with paraffin and incubate in dark at 25°C.
6. Observe under binocular.
7. After two weeks, transfer embryos to tubes with same medium without glutamine and only 2% sucrose.
Microspore Culture or Anther Culture:
By culturing haploid single celled pollen grains, haploid plants can be obtained. Though it is similar to protoplast culture, these two are fundamentally different in their original tissue as well as genetic constitution. The microspore is a true haploid cell system, which results in a more synchronized embryo development, thus eliminating the high somaclonal variation associated with protoplast.
This synchronised development of embryos helps in accurate mutation and selection methods. One of the advantages of this culture is that as much as 80% regeneration frequency can be obtained and the entire process upto plantlet regeneration takes only four weeks.
Haploid cultures are useful in plant breeding.
Requirements:
B 5 Medium:
Most laboratories use Nitsch and Nitsch (1967) medium after two modifications (don’t use this medium for isolation and washing). 0.05 mg/l BAP is optionally included in microspore medium to support normal embryo development.
1. Medium for embryos derived from microspore:
2. Donor plant. Brassica napus preferably grown in environmental chambers applied with NPK (20-10-20).
3. Two incubators (i) 25°C (ii) 32°C.
4. Dishes and blenders to excise and collect buds.
5. B5 medium for microspore isolation and washings. B5 medium for this purpose must be without phytohormones and with 13% (W/V) sucrose.
6. Sieve with 45 µm pore size.
7. Corning Petri plates (4″).
8. 6% Hypochlorite.
9. Swing bucket centrifuge.
Procedure:
1. Transfer sterile donor plants to lower temperature (10°C during day and 5°C during night) during bud harvesting. This cold treatment increases successive number of bud harvests and improves embryo yield and development.
2. Select buds with anthers having pollen at uninucleate stage.
3. Place selected buds in Petri plates/ beakers and immerse them in 6% hypochlorite solution for 15 minutes. Wash them 5-6 times with sterile distilled water to remove traces of hypochlorite.
4. Place these buds in a previously cooled (10°C) blender with approximately 0.5 to 1.0 ml of cold (10°-12°C) B5 medium and blend at high speed for 6-7 seconds- do not over-blend.
An alternative is to dissect buds in the medium, take out anthers and press them so that the microspores will come out.
5. Pass slurry from blender through 45 µm sieve and collect the suspension in a conical centrifuge tube.
6. Centrifuge filtrate at 250 rpm for 5-10 minutes in a swing bucket centrifuge tube.
7. Discard supernatant, resuspend pellet in B5 medium, wash and recentrifuge. Repeat the process three times.
8. Resuspend pellet in microspore medium and leave at 32°C, overnight.
9. Centrifuge (at 350 rpm for 10 minutes) and resuspend in fresh microspore medium.
10. Plate microspores in 4″ corning Petri plates with 2.5 to 3.0 ml microspore medium and incubate at 32°C. Several young torpedo-shaped and globular embryos appear after about 10-12 days.
11. Place these in the incubator at 25± 1°C with the addition of small amount of fresh microspore medium.
12. Transfer these to a slow (40-50 rpm) rotary shaker till torpedo-shaped embryos develop.
13. Transfer these plates with torpedo- shaped embryos to a rotary shaker in light at 25± 1°C.
14. Transfer these embryos to B5 medium (0.8% agar) with 2% sucrose.
True leaves are produced after two weeks.
15. Transfer them to pots. About 90% regeneration is obtained.
Isolation of Plant Protoplast:
In this process, the cell walls of plant cells are removed enzymatically. They retain all the cell organelles and nuclei and are totipotent and can be regenerated into plants using tissue culture techniques. Because of this, the protoplast is an ideal starting point for the gamut of genetic engineering technologies for plant improvement. Besides, protoplasts, are used in physiological investigations, ultra- structural studies, isolation of subcellular components like nuclei, chromosomes etc.
Principle:
The process consists of incubating tissues in a suitable mixture of cell wall degrading enzymes. The enzymes are then washed and the protoplasts are placed in culture medium.
The enzyme consists of a mixture of cellulase and pectinase. The protoplasts, when they emerge from the cell wall, has to be stabilized osmotically inorder to prevent them from bursting. This is done with either mannitol or sorbitol or glucose with concentrations varying from 0.3 (M) to 0.7 (M) depending on the source of protoplast e.g. Protoplasts from mesophyll requires an osmoticum of 0.5- 0.7 (M).
Requirements:
1. Nutrient medium:
Nagata and Takebe protoplast culture Medium (1971):
2. Donor:
Freshly cut tobacco leaves (Nicotiana tabacum) from plants grown in green houses.
3. Enzyme solutions:
Filter-sterilized and stored at -20°C
2.0% cellulyin
0.4% Macerease
0.7 M Mannitol
pH 5.7
4. Forceps, scalpel.
5. Pipettes and centrifuge tubes.
6. Centrifuge.
7. Petri plates.
8. 61 µm stainless steel filters.
Procedure:
A. Isolation of protoplast:
1. Immerse tobacco leaves in 95% ethanol for one minute, dry on sterilized surface of laminar flow cabinet.
2. Peal lower epidermis with sharp forceps or cut the leaf into 1 mm strips.
3. Pipette out 5 ml of enzyme solution into a sterile Petri plate and place the leaf / strips in it.
4. Place it on a shaker with gentle agitation.
5. Incubate overnight and observe release of protoplast.
B. Culture of protoplast:
1. Remove undigested leaf tissue (after incubation) and pour the suspension of protoplast through a 61 µm stainless steel filter.
2. Gently pour filtrate into sterile centrifuge tubes and centrifuge for 4 minutes at 500 rpm.
3. Pour out supernatant and add protoplast culture medium into the tubes.
4. Repeat centrifugation and washing with culture medium three times.
5. At the end of final wash, suspend protoplast in 2 ml culture medium and pour it into a 4″ (6 cm) Petri dish.
6. Seal the dish and observe protoplasts under an inverted microscope.
7. Place in an incubator and observe within a week, with calco fluor white-ST for wall regeneration.
Protoplast Fusion:
Principle:
In order to obtain interspecific fusion, a mixture of protoplasts from two different species is placed in polyethylene glycol solution and then eluted with high pH and Ca solution. For observing heterokaryosis green mesophyll protoplasts and colourless ones from cultured cells should be used.
Requirements:
Protoplast culture medium:
Solution ‘a’
Poly Ethylene Glycol (PEG) solution:
1. Protoplast from Carrot and Oats mesophyll tissue.
2. Petri dishes, slides, cover slips.
3. Mineral oil.
4. Pasteur pipettes.
5. Centrifuge tubes.
6. Scalpel etc.
Procedure:
1. Mix equal volumes of protoplast suspension of oats and carrot in culture medium.
2. Centrifuge for 5 minutes at 300 rpm and remove supernatant with Pasteur pipette.
3. Resuspend protoplast mixture in 10 ml of solution ‘a’.
4. Centrifuge and remove supernatant.
5. Resuspend protoplast mixture in one ml of solution ‘a’ to make a dense suspension.
6. Place a drop of mineral oil in a Petri dish and place a 22×22 mm cover slip on the drop.
7. Place a drop of protoplast suspension on the cover slip with a Pasteur pipette and allow the protoplast to settle down for 5 minutes and form a thin layer.
8. Add slowly, drop by drop, 30 drops of the PEG solution over the protoplast suspension.
9. Observe the phenomenon of adhesion under an inverted microscope.
10. Incubate the protoplasts in PEG solution at room temperature for 5-20 minutes.
11. Slowly start adding 0.5 ml aliquots of solution ‘b’ at 10 minutes interval.
After the second addition of solution, fusion of protoplasts start.
Cybrids:
These are fusion products of cells where one nucleus is eliminated. In the absence of selection pressure, the remaining chloroplast/mitochondrial mixture segregates randomly as the cell divides. This leads to creating different nuclear/chloroplast/mitochondrial combinations. These novel nucleocytoplasmic combinations are called cytoplasmic hybrids or ‘cybrids’.
Meristem Culture:
For a successful micropropagation, there are five (0-IV) critical stages:
Stage 0:
Selection of donor plant and preparation
Stage I:
Establishment of aseptic cultures
Stage II:
Proliferation of axillary buds/shoots
Stage III:
Pre-transplant (rooting).
Stage IV:
Transfer to natural environment.
In vitro culture of meristem is advantageous since it has maximum genetic stability. Even from infected plants-viral, bacterial or fungal pathogens can be eliminated.
Meristem is a small homogeneous tissue which provides a propagule for cryopreservation and other storage means of culture. These cultures fulfill quarantine regulations for international transport.
Requirements:
1. Culture medium-M.S. medium with selected growth regulators.
2. Donor plant Potato (Solanum tuberosum) Carrot (Dacus carota) Banana (Musa indica.)
3. Disinfectants-85% ethanol, sodium hypochlorite (0.5-10% V/V), a detergent (Tween 80, 1% V/V) and sterile distilled water.
4. Glasswares-Culture vessels.
5. Growth room with controlled conditions and a temperature of 25± 1°C.
6. Laminar clean air flow cabinet.
7. Scalpel, forceps, inoculating needles etc.
Procedure:
1. Excise terminal portion of stem or segments of stem with at least one bud.
2. Immerse the excised shoot segments in sodium hypochlorite solution (5-15 minutes based on the type of explant) of 0.5-10% (V/V) concentration. Add a drop of Tween 80 to the hypochlorite solution in order to wet the stem pieces.
3. Wash 5-6 times with sterile distilled water to remove all traces of hypo-chlorite.
4. Place the tissue on the stage of a dissecting microscope (15x) and dissect small developing leaves to expose apical meristem and a few young leaf primordia.
5. Transfer this to the selected growth mediums. Petri dishes should be sealed with paraffin to avoid evaporation of water.
6. Incubate in culture room (25± 1°C) with illuminations from 4000 Lux white fluorescent lamps with 12-16 hr. photoperiod.
7. Within 7-14 days of incubation, green colour and some elongation will be visible in case of viable plants.
8. Maintain these developing plants in vitro until the internodes are sufficiently elongated.
9. Dissect them into nodal explants and transfer to fresh original medium.
Within 7 days, growth of axillary buds will be visible.
Agrobacterium Mediated Transformation System:
There are two species of Agrobacterium:
1. A. tumefaciens which induces tumor in plants and
2. A.rhizogenes inducing proliferation of roots in a wide variety of dicotyledonous plants.
Both are soil inhabitants and enter plants through wounds only.
Transformation is the process of introducing DNA into cells and is important since DNA is the molecule that carries the blue prints of life. DNA can be cut or altered in vitro by isolating a single DNA sequence from one organism and joining it with that of a completely different organism to produce a recombinant DNA.
When a particular DNA sequence is to be transformed into a living cell, it is first inserted in a vector. Vectors are used to assist in the transfer, replication and sometimes expression of a specific sequence of DNA in a target cell.
But such vectors should have fairly small DNA molecules in order to help their isolation and handling. They must also have an origin of replication in order that their DNA can be copied and thus maintained in the population when the host divides and grows.
Agrobacterium tumefaciens, a soil inhabiting bacterium enters the host plant through a wound at the junction of stem and root (crown) causing gall formation. The agent that causes the gall is not the bacterium itself but the Ti plasmid. Ti plasmids are large 140-235 kb (1 kb = 1000 base pairs). When infection takes place, a small portion of Ti plasmid DNA (15- 30 kb) called T-DNA is transferred to the plant cell nucleus.
There it becomes covalently inserted into the nuclear DNA. T-DNA carries genes responsible for tumor formation and for the synthesis of unusual amino acid derivatives known as opines. The genes responsible for opine catabolism lie in the Ti-plasmid. Opines can be used as sole carbon and/or N2 sources for Agrobacterium which induces it.
There are different strains of Agrobacterium based on the type of opines that are synthesized by crown gall. Octopine and nopaline are the common types of opines. Ti plasmid contains also the genes responsible for the transfer of T-DNA and are called virulence genes(wV). The bacterium needs wounded tissues because vir genes are induced by phenolic compounds released by wounded tissues of plants. Thus, the transfer of T-DNA takes place when vir genes are induced.
It is only the border regions of T-DNA that are required for its transfer and integration into plant genomes and these include short repeat sequences of 25 base pairs. If DNA sequences are inserted between the border repeats, it will be transferred to and integrated into the genome of the plant. Hence to introduce foreign genes into plants, Ti-plasmids are good vectors.
For this, the genes responsible for tumor formation must be removed. Such Ti-plasmids lacking tumor forming capacity are known as ‘disarmed’ vectors. Plant cells infected by such plasmids do not produce tumors.
They can be regenerated into normal fertile plants. By this method, it is possible to insert specific DNA sequences with new desired characters to the recipient plants. However, such ‘disarmed’ plasmids are too large and hence smaller vectors based on features of Ti-plasmids can be constructed in vitro for easy manipulation.
Ti-plasmids have three oncogenes coding for enzymes involved in the synthesis of IAA and cytokinins.
Transformation with A. Tumefaciens based Vectors:
Principle:
A. tumefaciens which causes the crown gall of several dicotyledonous plants is used as a vehicle for gene transfer in plants and these transferred genes are stably integrated into the genomes of the transgenic plants which are transmitted to progeny.
Requirements:
1. Tissue culture.
2. A. tumefaciens strain with disarmed nopaline plasmid (helps in transforming plant cells to differentiate in a normal way without tumors).
3. Tobacco regeneration medium M.S. basal medium with:
NAA: 0.1 mg/l
BA: 0.2 mg/l
Sucrose: 30.0 g/l
Phytagel: 2.5 g/l
pH: 5.8 ammended with 100 mg/l Kanamycin and 200 mg/l Cefotaxime.
4. Two 125 ml corning flasks with 50 ml half strength M.S. medium.
5. Six 6 mm Petri dishes with half strength semisolid M.S medium.
6. Six 6 mm Petri dishes with tobacco regeneration medium.
7. Ten vessels with 75 ml of half strength M.S. medium supplemented with 100 mg/l Kanamycin and 200 mg/l Cefotaxime.
8. 40 ml GUS (P glucuronidase) assay buffer containing 0.1 M Phosphate buffer (pH 7.0) 5 mM potassium ferricyanide,5 mM potassium ferrocyanide and 10 mM EDTA.
9. 40 mg of x-gluc (5-bromo-4-chloro-3 indolyl-β-d-glucuronic acid).
10. 400 µl of N, N-dimethyl formamide.
11. Surface sterilant (sodium hypochlorite).
12. Hand gloves.
13. Centrifuge tube.
14. Stain for x-gluc staining.
15. Dissolve 40 mg x-gluc in 400 µl of N, N-dimethyl formamide.
When completely dissolved, add 40 ml of GUS assay buffer.
Place 10 ml of this solution each in four Petri dishes.
Procedure:
1. Place bacterial suspension into 50 ml centrifuge tube and centrifuge at 3000 rpm for 10 minutes and discard supernatant.
2. Suspend pellet in 10-15 ml ½ strength M.S. medium.
3. Aseptically remove leaves and place in Petri dishes.
4. Add bacterial suspension and cut the leaves aseptically into 2 cm pieces and submerge them in the bacterial suspension.
5. Incubate for 10 minutes.
6. Blot leaf segments on sterile filter paper (to remove and avoid growth of bacteria) and place them in Petri plates with ½ strength M.S. medium.
7. Seal Petri plates with paraffin and incubate at room temperature in dark for 2- 3 days during which T-DNA will be transferred from the bacterium to the leaf cells. The plant cells also now begin to express the gene which makes them resistant to Kanamycin selection.
8. After this transformation stage, transfer the leaves to plates containing tobacco regeneration (T.R.) medium with 100 mg/l Kanamycin and 200mg/l Cefotaxime. In this medium, Kanamycin will efficiently select transformed cells against untransformed ones. However, Agrobacterium cells also contain Kanamycin resistant genes but Cefotaxime will inhibit their growth.
9. Place Petri plates in culture room or incubator with 75-100 µ. mol m-2 sec-1 light and 20°-25°C temperature.
10. After 4-6 weeks, small amount of callus develop and shoots arise from cut edges of leaves.
11. When shoots become 0.5-1.0 cm in height, transfer them to nagneta boxe containers at the rate of 9-10 shoots per box with half strength M.S. medium plus 100 mg/l Kanamycin and 200 mg/l Cefotaxime, taking care to see that no callus tissue accompanies the plants.
12. After 3-4 weeks, roots start developing (white shoots which may appear are “escapes” or untransformed ones).
13. After 5-6 weeks, Kanamycin resistant plants will be seen as healthy green plants.
14. At this stage, plants can be examined for GUS expression.
(If seeds are obtained from these plants, place them in pots with soil mixture of perlite and bacto soil 1 : 3)
15. Remove plants with roots, clean them off agar, gently, and plant them in previously watered pots and cover them with inverted vessel to prevent wilting.
16. Water the plants and when buds appear, cover them with paper bags to prevent cross pollination.
17. Harvest seeds when dry.
18. To test for transformation and expression of the introduced genes into tobacco plants, X-gluc staining method is used. X-gluc is a colourless substrate that is cleaved by the GUS enzyme. The product of this enzymatic reaction forms a blue indigo dye that precipitates at the site of the enzyme cleavage. Blue colour permits the histochemical localisation of GUS activity and indicates which cells, tissues and organs express the GUS gene.
19. Method of staining:
a. Dissolve 40 mg. x-gluc in 400 µl of N-N-dimethyl formamide.
b. When completely dissolved, add 40 ml of GUS assay buffer.
c. Place 10 ml of this solution each in four Petri dishes,
d. Cut small (1-2 cm) pieces of:
(i) Young and old roots,
(ii) Young and fully expanded leaves,
(iii) Thin cross sections at different points along the stem and
(iv) L.S. of buds.
e. Immerse the tissues in assay solution.
f. Incubate for 1 hour at 37°C.
g. Check for colour development under high power dissection microscope.
h. If blue colour is still not visible, seal Petri plates and allow reaction to continue overnight at 37°C.
i. If chlorophyll masks blue colour immerse tissues in a 1:50 dilution of commercial bleach for 20 minutes to remove green chlorophyll.
j. Observe distribution of blue staining under microscope or take thin sections and observe.
Induction of Hairy Roots by Agrobacterium Rhizogenes:
Infection by the soil bacterium A. rhizogenes induces proliferation of roots in a large number of dicotyledonous plants, when it enters through a wound. This bacterium harbours large root inducing (Ri) plasmids. This bacterial plasmid DNA, when integrated into the host cell, a transformed phenotypic plant arises.
Like the Ti-plasmids of A. tumefaciens, the Ri-plasmids also have:
(i) The transferred or T. DNA that gets incorporated into the plant genome,
(ii) The vir or virulence region (essential for successful transformation) and
(iii) A region coding for the catabolism of opines (amino acid derivatives used as C and N2 source for bacteria). A. rhizogenes synthesizes the opines agropine, mannopine, or cucumopine. The T regions of DNA has TL and TR– DNA with rhizogenic functions and it has been suggested that TL-DNA is more important in hairy root induction.
Hairy root cultures are important in the studies on obligate root parasites or symbionts like mycorrhiza which cannot be grown in vitro. Root cultures are also useful for the production of secondary metabolites.
Requirements:
1. Yeast extract mannitol agar:
Yeast extract: 0.4 g
Mannitol: 10.0 g
k2HPO4: 0.5 g
MgS04.7H2O: 0.2 g
NaCl: o.1g
Agar: 15.0 g
Distilled water: 1000.00 ml
Ph: 7.0
2. Autoclave at 121°C (15 lb pressure) for 15 minutes.
3. Culture of A. rhizogenes on YEMA.
4. YEMA broth.
5. Beet root (Beta vulgaris).
6. Tobacco (Nicotiana tabacum).
7. Tissue culture medium: M.S medium with sucrose 30 g/l and pH 5.7 and for agar medium 6 g/l agar autoclaved at 121°C (15 lb pr) for 15 minutes.
8. Ampicillin in 1 NaOH (Filter sterilized in 0.22 µm millipore filter) and added to M.S. medium just when it is warm or above solidification temperature of 40°C after autoclaving.
9. Sterile 6 cm Petri dishes, 250 ml corning flasks closed with two layers of aluminium foil. Sterile flasks at 160°C for 90 minutes in an oven, cool and add medium.
10. Sodium hypochlorite.
11. Shaker.
12. Needles, forceps, inoculating loop etc.
13. Petri plates.
14. Pasteur pipettes.
Procedure:
1. Surface sterilize seeds with sodium hypochlorite solution (20 min) wash 5-6 times with sterile distilled water.
2. Make a thin layer of these seeds over agar based MS medium in 250 ml closed flasks and incubate at 25°C in light.
3. Seeds germinate in 10 days. Allow them to grow for 2-3 weeks (6 cm tall plants with 3-4 leaves).
4. Grow A. rhizogenes in YM broth on a shaker at 35°C (100 rpm).
5. Centrifuge culture for 10 minutes, remove supernatant with sterile pasteur pippette.
6. Resuspend pellet in YEMA Broth (small volume).
7. Scratch the stem of the plant above agar, with needle and smear bacterial suspension in the scratches taking care to see that bacteria will not touch the agar medium.
8. Close the flasks and incubate at 25°C within 7-14 days hairy roots start growing from the scratches.
9. Remove roots aseptically and transfer them to M.S. medium with ampicillin in Petri plates.
10. Seal Petri plates and incubate in inverted position at 25°C.
11. After 2-3 weeks remove 1-2 cm root tips and transfer them to fresh M.S. medium with ampicillin.
Hairy roots itself is an indication of their transformed nature. Direct confirmation of plasmid T-DNA in the hairy roots can be done by southern blotting and hybridisation to a labelled probe prepared from plasmid T-DNA.
Suspension Cultures-Induction of Embryogenesis:
Somatic embryos arise from differentiated tissues in plant parts grown in vitro. Somatic embryos, in contrast to zygotic embryos, have a bipolar axis with an apical meristem and root pole. They have no vascular connection with the mother callus and hence can easily be detached.
In economically important plants especially of the family Poaceae, e.g. Zea’ mays Triti- cum vulgare, etc. somatic embryogenesis is the most common method of regeneration of plants.
Three types of media are required for this purpose:
i. Solidified agar medium with auxin to differentiate somatic tissue and induce embryogenic cells (primary culture).
ii. Liquid medium with auxin to ensure multiplication of these cells.
iii Liquid medium without auxin to allow the cells to express their embryogenic potential.
Liquid cultures need agitation. Otherwise cells sink to the bottom and die. Swirling separates embryos and they can be fractionated based on their stages and can be obtained in large quantities.
Requirements:
1. M.S. medium with pH 5.5.
2. ½ M.S. medium with 5 g/l sucrose.
3. Actively growing callus (Phaseolus).
4. Growth chamber.
5. Culture room.
6. 1 mm grid stainless steel sieves.
7. Filter paper bridge set up.
Procedure:
1. Grow explant (Phaseolus) on M.S. solid medium with 1mg/l 2,4-D.
2. Callus develops after 5 weeks.
3. Transfer this callus to liquid medium. After removing brown regions, approximately 2 g is inoculated in 250 ml flasks with 50 ml M.S. liquid medium and is kept on a rotary shaker at 100-150 rpm.
4. Transfer to fresh medium after 2-3 weeks since the cells after a lag period and an exponential rise in cell number reach a stationary phase.
5. This transfer should be made at regular intervals maintaining a minimum density which helps in maintaining embryogenic potential.
6. Sub-culturing should be done after all the cells settle down and then the entire medium should be decanted.
7. Gently rotate, resuspend cells and transfer one fourth to fresh medium in flasks.
8. This will have all stages of embryo development e.g. single cells, pro-embryonic cell clusters, new embryo-genic centers and older embryos. In order to have uniformity, sieve inoculum while transfering.
9. Pro-embryonic suspension is passed through a 200 µm and then a 100 µm sieve. In 100 µm sieve, differentiated embryos are retained.
10. Transfer immature embryos to liquid medium with same composition without auxin and glutamine. They take two weeks to mature.
11. Disperse them in Petri dish on agar medium without auxin but with small amount of (0.1 mg/l) of BAP or Zeatin.
12. Transfer mature embryos to filter paper bridges in tube’s wet with ½ M.S. and 5 g/l sucrose (liquid) medium and incubate in light.
13. Transfer to pots with vermiculite + peat + soil and cover pots with plastic bags.
Dual Cultures – Legume Rhizobium:
Dual cultures are carried out in order to find out the amount of nitrogen fixed by Rhizobium in vitro. This will help in raising disease free plantlets which can be nodulated in vitro.
Requirements:
1. M.S. and Blayde’s medium supplemented with growth regulators:
Blayde’s (BLD) Medium (1966):
2. Lie’s (1971) modified N2 free medium:
3. Seeds of Phaseolus.
4. 90% alcohol, 0.1% HgCI2
5. Rhizobium culture.
6. Scalpel, inoculating needles, etc.
7. Petri plates, test tubes, etc.
8. Sterile distilled water.
Procedure:
1. Wash seeds of Phaseolus in running tap water and then place them in 90% alcohol for 2 minutes.
2. Wash and place them in 0.1% HgCl2 for 5-10 minutes and wash 5-6 times in sterile distilled water.
3. Incubate sterilized seeds in Petri plates with moist filter paper and germinate.
4. Cut aseptically, explants (0.5-0.7 cm) from 2-4 days old Phaseolus seedlings an inoculate on BLD and M.S. medium supplemented with growth regulators in 6″ X 1″ tubes.
5. Subculture calli that developed on N2 free M.S. and BLD media.
6. Make a thick suspension of 24-48 hour old YEMA grown authentic strain (isolated from Phaseolus and authenticated by reinoculation) of Rhizobium and inoculate 0.1 ml on the callus. The callus turns brown due to Rhizobium infection in about 4-5 days and seedlings develop. In callus with seedlings when inoculated with Rhizobium, nodule like structures develop.
7. Squash the brown calli and nodules, stain them either with cotton blue or acetocarmine and observe under the microscope.
8. After incubating in dark, measure nitro- genase activity from 6-15 days by acetylene reduction test.
Chloroplast Isolation:
Chloroplasts are isolated, to study electron transport system of the photosynthetic apparatus.
Principle:
Various organelles of a cell sediment at different centrifugal fields, depending upon their size and weight.
Requirements:
1. Isolation medium:
2. Spinach leaves.
3. Homogeniser.
4. Cheese cloth.
5. Centrifuge.
Procedure:
1. Cut 10 g spinach leaves into small pieces and homogenise with 20 ml of pre chilled isolation medium in a mixer (3-5 seconds) and filter through 8 layered cheese cloth.
2. Centrifuge filtrate at 3000 rpm for 2 minutes and discard supernatant.
3. Resuspend pellet in small volume of grinding medium and store on ice.
Estimation:
Dilute 0.1-0.2 m of chloroplast suspension to a total volume of 4 ml with 80% acetone.
Calculation of chloroplast content:
Estimation of Chlorophyll:
Chlorophyll, the essential component of photosynthesis in plants contains a porphyrin (tetrapyrole) nucleus with a chelated Mg atom at the centre and a long chain hydrocarbon (phytyl) side chain attached through a carboxylic acid group.
Chlorophyll a and b – Found in higher plants, mosses and ferns.
Chlorophyll c and d – Found in algae and certain bacteria.
Principle:
Chlorophyll is extracted in 80% acetone and absorption at 663 nm and 645 nm are read in a spectrophotometer. The amount of chlorophyll is calculated using the absorption coefficients.
Requirements:
1. 80% chilled acetone.
2. Spinach leaves.
3. Mortar and pestle.
4. Centrifuge.
5. Spectrophotometer.
Procedure:
1. Grind 1 g finely cut spinach leaves to a fine pulp with 20 ml of 80% chilled acetone in a mortar and pestle.
2. Centrifuge at 5000 rpm for 5 minutes and transfer supernatant to a 100 ml volumetric flask.
3. Grind the residue with 20 ml 80% chilled acetone, centrifuge and transfer supernatant to the volumetric flask. Repeat this until the residue is colourless.
4. Wash mortar and pestle with acetone until washings are colourless and collect them.
5. Make up the volume to 100 ml with 80% chilled acetone.
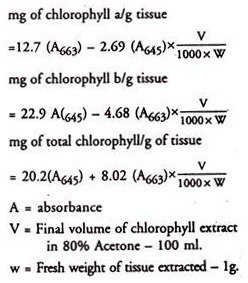
6. Read absorbance at 645, 663 and 652 nm against 80% acetone blank.
Calculation: