In this article we will discuss about the isolation and authentication of rhizobium.
Isolation of Rhizobium:
Rhizobium is a symbiotic N2 fixer found to occur as bacteroids in the root nodules of leguminous plants. They can be easily isolated and cultured in vitro. Rhizobia are Gram- negative rods which are motile with bi-polar, sub-polar and peritrichous flagella.
Principle:
Rhizobium grows well on Yeast Extract Mannitol Agar (YEMA). Congo red added to the medium differentiates rhizobia stand out as white, translucent, glistening elevated, small colonies with entire margin, in contrast to the red stained colonies of Agrobacterium.
Requirements:
1. Root nodules (pink) of Phaseolus.
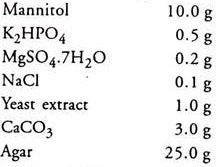
2. Congo red, Yeast Extract, Mannitol Agar (pH 6.8 – 7.0):
Congo red (1% aqueous)
2.5 ml (1.0 g in 100 ml)
Distilled water 1000.0 ml
3. Inoculation loop/needle.
4. Bunsen burner/laminar clean air flow hood.
5. Slides and glass rod.
6. Petri plates and tubes with YEMACR.
7. Sterile distilled water.
10. 95% alcohol and 0.1% HgCl2.
Procedure:
1. Loosen the soil around an actively growing Phaseolus plant about 14-16 cm away and uproot it slowly.
2. Wash the root system under a slow stream of running tap water, taking care to see that the nodules are intact.
3. Select pink nodules, remove them by keeping a bit of root on either side.
4. Wash and keep the nodules in 95% ethanol for a minute, wash and transfer them to 0.1% HgCl2.
5. Remove after five minutes and wash the nodules about four to five times with sterile distilled water.
6. Place the nodule on a sterile slide in a drop of sterile distilled water and crush it either with a sterile glass rod or a flat tipped forceps (plucker).
7. Remove a loopful of this cloudy suspension and streak inoculate on YEMACR plates and label.
8. Incubate in dark at 28°-30°C for 2-3 days.
9. Mucoid colonies which do not take up congo red appear on the agar medium.
10. Subculture them on YEMACR slants and store for authentication and further use.
Authentication of Rhizobium:
Authentication is the main test to confirm the identity of the isolate for which Leonard jar Assembly (Fig. 2.4) method of Vincent (1970) is used.
Principle:
Compatible strains isolated will form effective nodules that fix nitrogen. Leonard Jar Method.
Requirements:
1. Amber coloured beer bottles cut across into two halves.
2. Sterile absorbent cotton.
3. Lamp wicks.
4. Washed oven dried sand.
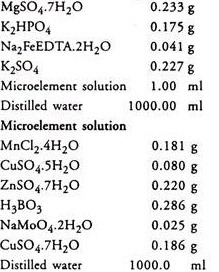
5. Reading’s N2 free solution (pH 6.5-7.0):
6. Culture of Rhizobium from Phaseolus.
7. Seeds of Phaseolus.
Procedure:
1. Invert upper half (neck) of the beer bottle in lower half after plugging the neck with a absorbent cotton. The plug should have in, the centre, the wick of a lamp one half of the wick should be in the upper and the other in the lower half of the bottle assembly.
2. Holding the wick in the centre, fill the upper half of the assembly with oven dried sand, pH of which being adjusted with CaC03.
3. Fill the reservoir (lower half) with ¼ th strength, Readings N2 free medium.
4. Cover the top with one half of a Petri plate and autoclave the entire assembly at 15 lbs (121°C) pressure for two hours.
5. After cooling make a hole 3 cm deep in the centre of the sand, remove it and keep it aside.
6. Place 1 ml of 72-hour-old culture of Rhizobium isolated from Phaseolus in the hole.
7. Place surface sterilised pregerminated seed of Phaseolus raised in rooting medium in the hole over Rhizobium culture and place the original soil (3 cm) that was removed and kept aside, over the seeds.
8. Cover the open end of the assembly with one half of the Petri dish and leave it in culture room for four to six weeks.
9. Remove the Petri dish when the seedling starts growing above the sand level.
10. Collect plants after four to six weeks.
11. Note the number of nodules and their size.
12. Record your observations.