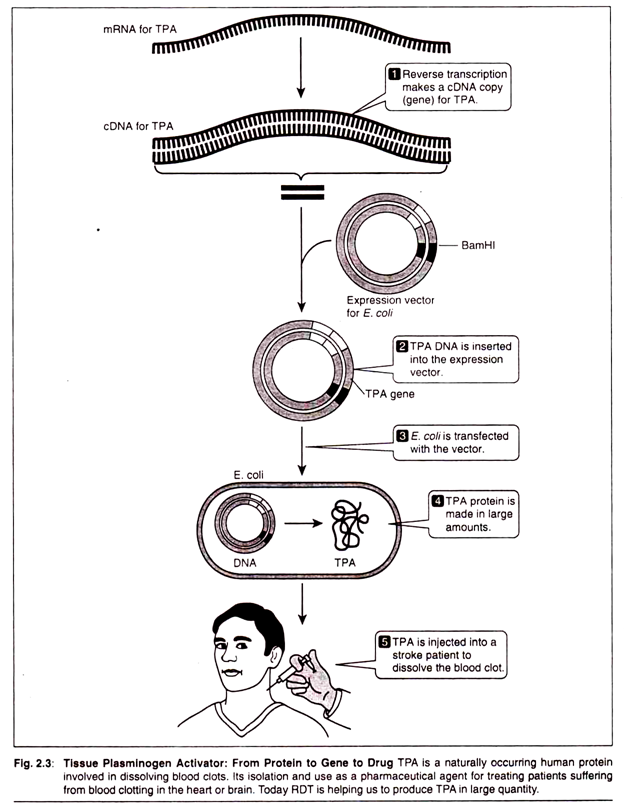
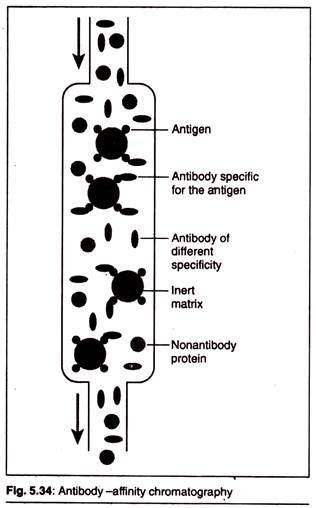
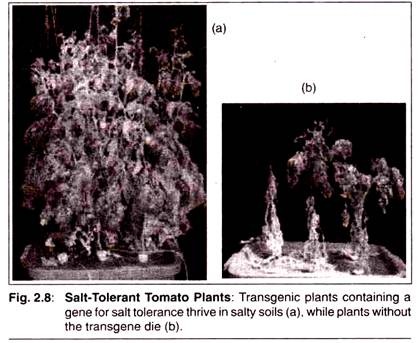
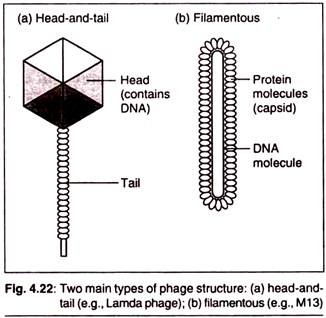
The following points highlight the top eight biochemical tests for bacteria. The tests are: 1. Carbohydrate Fermentation 2. Triple Sugar Iron Agar Test 3. IMVIC Test 4. Hydrogen Sulphide Test 5. Urease Test 6. Catalase Test 7. Oxidase Test 8. Nitrate Reduction Test.
Biochemical Test # 1. Carbohydrate Fermentation:
Different microorganisms utilise carbohydrates in various ways. Some ferment sugars like glucose anaerobically while others do it aerobically. Facultative anaerobes produce enzymes to cause fermentation both aerobically and anaerobically.
Principle:
Facultative anaerobes ferment glucose by Emden-Meyerhof pathway (Glycolysis) and produce acid and gas (CO2). Acid will turn phenol red, yellow and CO2 will evolve and get collected in the Durham’s tube.
Requirements:
1. Test medium:
2. Durham’s tubes.
3. Inoculating loop.
4. Test organism Escherichia coli, Staphylococcus.
5. Bunsen flame.
6. Phenol red.
7. Test tubes with different concentrations of sugar solution.
8. Incubator.
9. Glass marking pencil.
Procedure:
1. Distribute different concentrations of sugar solution in tubes with inverted Durham’s tubes and autoclave.
2. After cooling inoculate loopfuls of test organisms in tubes with different concentrations of sugar and incubate at 37°C for 24-48 hours.
3. Add phenol red and observe the broth for colour and presence or absence of gas in Durham’s tubes. If broth turns yellow and if gas is produced, it is a positive reaction.
Biochemical Test # 2. Triple Sugar Iron Agar Test:
This test is done to differentiate members of Enterobacteriaceae and also to distinguish between Enterobacteriaceae (which are all Gram-negetive bacilli capable of fermenting glucose with production of acid) and other groups of intestinal Gram-negetive bacilli.
Principle:
This differentiation is made on the basis of differences in the fermentation patterns of carbohydrates and H2S production by different groups of intestinal organisms. In order to observe carbohydrate utilisation patterns, TSI agar slants have 1% concentrations of sucrose and lactose and 0.1% of glucose which allows utilisation of these substrates only. Incorporation of acid-base indicator, phenol red, helps in detecting carbohydrate fermentation which can be observed by change in colour of the medium from orange-red to yellow when acids are formed.
Requirements:
1. Test organisms Escherichia coli, Pseud- omonas aeruginosa on nutrient agar slants.
2. Triple sugar; Iron agar (pH 7.4):
3. Glass-wares.
4. Inoculating needles/loops.
5. Bunsen flame.
6. Glass marking pencil.
7. Incubator.
Procedure:
Stab and streak inoculate test organism into TSI slants and incubate at 37°C for 18-24 hrs.
Observe:
Biochemical Test # 3. IMVIC Test:
Members of the Enterobacteriaceae are a group of Gram-negative, non-spore forming bacilli found in the intestinal tract of humans and lower mammals, some of which are pathogenic.
They can be differentiated on the basis of their biochemical properties and enzymatic reactions in the presence of substrates.
The IMVIC Series of tests include:
(i) Indole production test,
(ii) Methyl red test,
(iii) Voges- Proskauer, and
(iv) Citrate utilisation tests.
(i) Indole Production Test:
This test determines the ability of the organism to degrade the amino acid tryptophan.
Principle:
Enzyme tryptophanase produced by the organism converts tryptophan into indole, pyruvic acid and ammonia. This is not characteristic of all organisms and hence can be used as a biochemical marker.
Requirements:
1. Tubes with SIM agar having Tryptophan as substrate:
2. Kovac’s Reagent:
Dissolved-dimethyl aminobenzaldehyde in amyl alcohol and then add HCl.
3. 24-48-hour-old cultures of Escherichia coli, Proteus vulgaris, Enterobacter aerogenes, etc. in Tryplicase Soya Broth:
Procedure:
1. Stab inoculates organisms into SIM agar along with controls and incubate at 37°C for 24-48 hours.
2. Add Kovac’s reagent:
A cherry-red coloured reagent layer which is composed of p-dimethyl aminobenzaldehyde, butanol and HCl appear. Acidified butanol component, extracts indole from the medium into the reagent layer and forms a complex with p-dimethyl aminobenzaldehyde yielding the cherry-red colour. When this cherry-red colour appears, it means that the organisms produce indole, degrading tryptophan and is positive whereas if no red colour is produced, it is negative.
(ii) Methyl Red Test:
Principle:
All enteric organisms oxidise the hexose monosaccharide, glucose and produce organic acid. The end product depends on the specific enzymatic pathways present in bacteria. All enteric bacteria that oxidise glucose to produce high concentrations of acids, their end products can be differentiated, e.g. in case of bacteria like Escherichia coli and Enterobacter aerogenes it is so.
Requirement:
1. MRVP Broth (pH 6.9):
2. Methyl red indicator:
Dissolve methyl red in alcohol and make up the volume to 500 ml with distilled water.
3. 24-48 hour cultures of Escherichia coli, Enterobacter aerogenes on trypticase soya broth or nutrient agar/broth.
4. Inoculating loop.
5. Bunsen flame.
6. Glass marking pencil.
7. Incubator.
Procedure:
1. Inoculate organisms aseptically into the tubes with medium along with controls and incubate at 37°C for 24-48 hours.
Both E. coli and E. aerogenes produce organic acid as end product during early incubation. During later stages, E. aerogenes converts these acids enzymatically into non-acidic end products like 2, 3 butanediol and acetoin (acetyl methyl carbinol) leading to an elevated PH6.
2. Methyl red indicator at pH4 will turn red which is a positive test.
3. At pH6 indicator turns yellow which is a negative test.
4. The production of non-acidic end products by Enterobacter aerogenes from glucose fermentation and their detection is amplified by Voges-Proskauer test which is carried out simultaneously with methyl red test.
(iii) Voges-Proskauer Test:
This test is carried out to further differentiate among enteric forms like E.coli, Enterobacter aerogenes and Klebsiella pneum- oniae. It determines the ability of some of these organisms to produce non-acidic or neutral end products like acetylmethyl- carbinol from organic acids that result from the metabolism of glucose by forms like Enterobacter aerogenes.
Principle:
The Barritt’s reagent is a mixture of alcoholic alfa-naphthol and 40% KOH solution. Acetylmethylcarbinol is to be xidized to a diacetyl compound in order that it can be detected. This reaction occurs in presence of alfa-naphthol catalyst and a guanidine group that is present in the peptone of MRVP broth. A pink complex formed will impart rose colour to the medium.
Requirements:
1. Barritt’s Reagent:
Solution A:
Alfa-naphthol – 5.0 g
Absolute alcohol – 95.0 ml
Dissolve by constant shaking/stirring.
Solution B:
KOH – 40.0 g
Ceratine – 0.3 g
Distilled water – 100.0 ml
Dissolve KOH in 75 ml distilled water cool and then add ceratine and make up the volume to 100 ml.
2. MRVP broth.
3. 24-48-hour-old cultures of E.coli, E. aerogenes and Klebsiella pneumoniae on trypticase soya broth/nutrient broth.
4. Aliquots of trypticase soya broth cultures.
5. Inoculation loop.
6. Bunsen flame.
7. Laminar clean air flow hood.
8. Glass marking pencil.
9. Incubator.
10. Methyl red.
Procedure:
1. Inoculate 1/3 culture of test organisms Escherichia coli, Enterobacter aerogenes and Klebsiella pneumoniae into empty tubes and keep them aside for this test (Voges-Proskauer test).
Methyl red test:
Add 5 drops of methyl red to the remaining culture and examine the colour of each culture and note.
2. To these broth cultures ( 1/3 culture ) add 10 drops of Barritt’s reagent A’ and shake the cultures immediately followed by 10 drops of Barritt’s reagent ‘B’ and shake. Shake the tubes again and again every 3-4 minutes.
3. After 15 minutes, observe the colour. If the medium becomes deep rose in colour after 15-20 minutes, it is a positive reaction where the organism was capable of fermenting glucose with the ultimate production of acetyl methyl carbinol.
(iv) Citrate Utilisation Test:
Some microorganisms utilise citrate as carbon and energy source in the absence of fermentable sugars like glucose. Presence of a citrate permease in organisms helps in the transport of citrate into the cells. In Kreb’s cycle, citrate is the first intermediate which is acted upon by citrase—> oxalic acid and acetate which are enzymatically converted to pyruvic acid and CO2. At this time, the medium becomes alkaline (CO2 combines with Na and H2O to form Na2CO3).
Principle:
The Na2C03 (alkaline) produced in the medium due to utilisation of citrate, changes the indicator bromothymol blue from deep green to deep prussian blue.
This test differentiates enteric forms that ferment citrate and use that as the sole source of carbon and energy.
Requirements:
1. Fresh cultures of E. coli, E. aerogenes and K. pneumoniae.
2. Simmon’s citrate agar (pH 6.9):
3. Inoculation loop/needle.
4. Bunsen flame.
5. Test tubes.
6. Glass marking pencil.
7. Incubator.
Procedure:
1. Aseptically stab and streak inoculate organisms into Simmon’s citrate agar slants.
2. Incubate the cultures at 37°C for 24- 48 hours and observe.
Tubes of cultures that have become prussian blue show positive reactions.
Biochemical Test # 4. Hydrogen Sulphide Test:
Organisms produce H2S from inorganic sulphur compounds or from sulphur containing amino acids. H2S is produced by some organisms by two major fermentative pathways (i) Organic S present in cysteine (present in peptone, a component of the medium), formed after decomposition of peptone, is degraded by cysteine desulfurase. The sulphur, thus released is reduced by H2 present in water to form H2S gas. (ii) Inorganic sulphur compounds like thiosulphates (S2O3), sulphates (SO4) and sulphites (SO3) present in the medium are reduced with the liberation of H2S.
Principle:
In SIM agar peptone (cysteine) Na2S2O3 and ferrous ammonium sulphate are the ones with sulphur in this semi-solid (3 g agar per 1) medium. Ferrous ammonium sulphate in the medium combines with H2S and forms an insoluble black precipitate of ferrous sulphide along the line of stab of inoculum.
Requirements:
1. 24-48 hour cultures of Enterobacter aerogenes, Proteus vulgaris and Salmonella typhimurium.
2. SIM Agar tubes:
SIM agar (pH 7.3):
3. Bunsen flame.
4. Inoculating needle.
5. Glass marking pencil.
6. Incubator.
Procedure:
1. Stab inoculates the organisms into SIM agar tubes along with controls.
2. Incubate at 37°C for 24 to 48 hours. Formation of a black insoluble precipitate along the line of stab is a positive result.
Biochemical Test # 5. Urease Test:
Urease, a hydrolytic enzyme, attacks the nitrogen and carbon bonds in amide compounds like urea and forms an alkaline end product, ammonia. This is detected by a pH indicator phenol red which will be turned to deep pink.
Principle:
When organisms are grown in urea broth containing phenol red (pH indicator), the end product is ammonia formed as a result of the splitting of substrate urea. This can be detected by the change in colour of phenol red to deep pink.
Requirements:
1. Cultures of Proteus vulgaris and E. coli.
2. Tubes with urea broth:
Add filtered broth concentrate of urea to 90 ml cooled sterile distilled water and add 3 ml of this into sterile tubes.
3. Inoculation loop.
4. Bunsen flame.
5. Glass marking pencil.
6. Incubator.
7. Phenol red.
Procedure:
1. Loop inoculates organisms into urea broth.
2. Incubate at 37°C for 24-48 hours. Appearance of deep pink colour after addition of phenol red is a positive reaction.
Biochemical Test # 6. Catalase Test:
Microorganisms, during aerobic respiration produce H2O2 and extremely toxic superoxide which are harmful, leading to its death. Such toxic substances are produced when aerobes, facultative anaerobes and microaerophiles use aerobic pathway of respiration where O2 is the final electron acceptor during carbohydrate degradation. Organisms producing catalase, degrade H2O2
Superoxide dismutase degrades toxic superoxides in catalase negative organisms. For strict anaerobes O2 is poisonous since they cannot produce catalase, peroxidase or superoxide dismutase.
Principle:
To a culture if the substrate is H2O2 added, bubbles of free O2 gas is evolved.
Requirements:
1. Cultures of Staphylococcus aureus, Micrococcus luteus and Streptococcus lactis.
2. Trypticase soy agar slants:
Trypticase soy agar (pH 7.3):
3. 3% H2O2
4. Inoculation loop/needle.
5. Bunsen flame.
6. Glass marking pencil.
7. Incubator.
Procedure:
1. Streak inoculate test organisms on Trypticase soy agar, incubate at 37°C for 24-48 hours. Then add 3% to H2O2 tubes. In positive organisms, O2 bubbles will evolve.
Biochemical Test # 7. Oxidase Test:
Oxidases play an important role in the operation of electron transport system during aerobic respiration. The oxidation of a reduced cytochrome by molecular 02 is catalyzed by cytochrome oxidase leading to formation of H2O2 and H2O. This test differentiates members of the genera Neisseria and Pseudomonas which are oxidase positive and the oxidase negative Enterobacteriaceae respectively.
Principle:
The test reagent p-aminodimethylaniline oxalate is added to colonies in plates. This (pink reagent) acts as an artificial substrate donating electrons and thereby becomes oxidised to a blackish compound in presence of oxidase and free oxygen.
Requirements:
1. Cultures of E. coli, Pseudomonas aeruginosa and Acaligenes faecalis.
2. Trypticase soy agar plates.
3. p-aminodimethylanilene oxalate.
4. Inoculation loop.
5. Bunsen flame.
6. Glass marking pencil.
7. Incubator.
Procedure:
1. Using glass marking pencil, divide trypticase soy agar into three at the bottom of the Petriplates.
2. Aseptically streak three different cultures on the three different sectors label and incubate at 37°C for 24-48 hours in inverted position.
3. Add light pink coloured p-aminodi- methylaniline oxalate reagent.
The colour of the reagent pink will change —> maroon —> black.
Biochemical Test # 8. Nitrate Reduction Test:
Some microorganisms reduce nitrates (NO3–) to nitrite (NO2–) or beyond nitrite stage. This anaerobic respiration is an oxidative process wherein organisms use nitrates or sulphates to obtain oxygen that is subsequently utilised as a final hydrogen acceptor during energy formation.
Principle:
The organisms are grown on a semisolid (0.1% agar) with 0.1% KNO3 as substrate. O2 diffuses into the semisolid medium and favours nitrate reduction. Addition of sulfanilic acid and alpha naphthylamine will produce a cherry-red colour as positive reaction.
Requirements:
1. Cultures of E.coli, Acaligenes faecalis and Pseudomonas aeruginosa.
2. Trypticase nitrate (pH 7.2):
3. Reagents:
4. Petri plates, test tube.
5. Bunsen flame/Laminar clean air flow hood.
6. Glass marking pencil.
7. Inoculating loop.
8. Incubator.
Procedure:
1. Inoculate the test organisms along with controls into Trypticase nitrate medium and incubate at 37°C for 24-48 hours.
2. Add sulfanilic acid and alpha naphthylamine to cultures. Cherry-red colour is a positive reaction. If no colour is produced, it might either be because nitrates were not reduced or that NO3 reductase was so potent that it reduced NO3 rapidly beyond nitrites to NH3 or even molecular N2.