Interview Questions and Answers for Fresher’s In Biotechnology!
Q.1. What is DNA?
Ans. In most living organisms, DNA is the genetic material except for some viruses such as the AIDS and the Tobacco mosaic virus where RNA is the genetic material. DNA and RNA belong to a category of macromolecules known as nucleic acids. The structure of DNA was proposed by Watson and Crick in the year 1953.
A DNA molecule is a long chain polymer composed of monomeric units called nucleotides. Therefore, a DNA molecule is made of a polynucleotide chain. Each nucleotide is made of a deoxyribose sugar molecule, a phosphoric acid group and nitrogen bases.
Q.2. What are the important features of DNA revealed by Franklin and Wilkins?
Ans. The important features of DNA revealed by Franklin and Wilkins are as follows:
a. It has a helical structure similar to a spiral staircase.
b. The molecule has a diameter of 20 Å.
c. The distance between successive nucleotides is 3.4 Å.
d. The distance between successive turns of the helix is 34 Å, i.e. there are 10 nucleotides in every turn of the helix.
Later in the year 1950, Erwin Chargaff formulated important generalisation on the ratio of the different bases found in DNA.
These generalisations are referred to as Chargaff’s rules, which are as follows:
a. In a given DNA, the purines and pyrimidines are always in equal amounts, i.e. adenine (A) + guanine (G) = thymine (T) + cytosine (C).
b. The amount of adenine is always equal to that of thymine, and the amount of guanine is always equal to that of cytosine.
c. The sugar, deoxyribose, and phosphate occur in equimolar proportions
d. The ratio of A + T / C + G is constant for a species, which can be used to identify the source of DNA.
With these two lines of evidences explained above, Watson and Crick proposed the double helical structure of DNA and they were awarded the Nobel Prize for this discovery in the year 1962. They shared the award with Maurice Wilkins. The double helical structure could explain the two main functions of DNA, i.e. the autocatalytic and the hetero catalytic functions very convincingly.
Q.3. What do you mean by DNA Isolation?
Ans. The physiological chemist, Miescher (1844-1895), from Basel in Switzerland, is recognized as the first scientist to discover DNA. It was while working on pus cells that Miescher made this fundamental discovery.
It was thought that such cells were made largely of protein, but Miescher noted the presence of something that ‘cannot belong among any of the protein substances known hitherto’. He was able to show that it was not a protein at all because it was not digested by protease.
He also showed that the new substance was derived from the nucleus of the cell alone and consequently named it as nuclein. Miescher was later able to show that nuclein could be obtained from many other cells.
Albrecht Kossel eagerly took up Miescher s work on nuclein and succeeded in recognizing the four nucleic acid bases (A, T, C, G). Nuclein was later renamed nucleic acid by Richard Altmann in 1889; James Watson and Francis Crick revealed its structure in 1953.
The genome constitutes the total genetic information of an organism. The genomes of all organisms are DNA, except some viruses that have RNA as their genetic material. Genomic DNA molecules in most organisms are organised into protein-DNA complexes called chromosomes.
The size, number and nature of genomic DNA vary between different organisms. In almost all organisms the DNA is double stranded in nature. Bacteria have a single and circular chromosome. Viral DNA genomes are relatively small and can be single- or double-stranded, linear or circular.
In eukaryotes, most genomic DNA is located within the nucleus. Additionally eukaryotic cells contain genomic DNA in the mitochondria, and in plants and lower eukaryotes, the chloroplasts contain genomic DNA.
This DNA is usually a circular molecule and is present as multiple copies within these organelles. DNA is found in the nucleus in eukaryotic organisms and floating in the cytoplasm in prokaryotic organisms. It is a fact that DNA is found in all cells except for red blood cells.
The isolation of DNA is one of the more commonly used procedures in many areas of physiology, genetics, molecular biology and biochemistry. Purified DNA is required for many applications such as studying DNA structure and chemistry, examining DNA-protein interactions, carrying out DNA hybridizations, sequencing or polymerase chain reactions (PCR), and performing various genetic studies or gene cloning.
Q.4. How to isolate Fungal Genomic DNA?
Ans. Harvest mycelium directly from a culture disc or liquid culture. In the case of liquid cultures cells should be pelleted by centrifugation and the supernatant removed before DNA isolation or storage. Harvested samples (mycelia) can be either directly frozen or freeze dried and stored at -80°C.
1. Grow fungus in the medium (saboured dextrose broth) to obtain clear mycelial pad or mat.
2. Place 200 mg mycelial pad in a 5 ml disposable centrifuge tube, break up the mycelial pad with a spatula or glass rod. Add a pinch of glass powder or glass beads (3mm) and briefly shake the mycelial pads.
3. Add 4 ml of CTAB extraction buffer; gently mix to wet the entire powdered pad.
4. Place in a water bath at 65°C for 30 minutes.
5. Cool and add equal volume of chloroform-isoamyl alcohol (24:1).
6. Mix and centrifuge in a microfuge at 10,000 rpm for 10 minutes.
7. Collect the supernatant. Add equal volume of isopropanol.
8. Spool out the DNA with a glass rod or loop (or centrifuge at 10,000 rpm for 2-5 minutes).
9. Wash the DNA with 70% ethanol in a tube (or add 1 ml of 70% ethanol. Mix by inverting several times). Drain the tubes on a paper towel.
10. Air dry and add 200 μl TE containing 20 μg/ml RNase A. To re-suspend the samples, place in 65°C bath, or allow pellets to re-suspend overnight at 4°C.
11. Ethanol precipitate the DNA.
Fungal growth medium (saboured dextrose broth medium)-
Add a pinch of streptomycin to the culture medium after sterilization.
CTAB Extraction buffer (100 ml) (Cetyltrimethylammonium bromide)-
100 mM Tris – 1.214 g Tris base
1.4 M NaCl – 8.18 g
EDTA (disodium salt) – 1.11 g
2% (w/v) CTAB – 2.0 g
Allow some time for the CTAB to dissolve and heat it to 65°C. The buffer is usually pH 8.0 without any adjustment. Do not autoclave CTAB buffer.
Essential Techniques in Working with DNA:
a. Hybridization:
Denaturing the DNA and then adding another DNA with radioactive substance which has correct DNA sequence is called Hybridization probe.
It can be synthetic DNA or isolated DNA. To keep it original and single, it is attached to a nitrocellulose membrane.
b. Gel Electrophoresis:
DNA has a negative charge, and all DNA has a uniform mass to charge ratio moving in water by suing a gel with sieving action. DNA is separated on basis of size, detecting by UV florescence.
c. Southern Blotting:
It uses gel electrophoresis and hybridization techniques to detect DNA. Also the bacterial colonies or plaques with DNA are detected by this method.
d. DNA Sequencing:
A dNTP is used to produce a ladder of DNA fragments that terminate only at specific nucleotide. Four ladders are used.
Q.5. What do you understand by the term DNA Recombination?
Ans. A DNA helix usually does not interact with other segments of DNA, and in human cells the different chromosomes even occupy separate areas in the nucleus called “chromosome territories”. This physical separation of different chromosomes is important for the ability of DNA to function as a stable repository for information, as one of the few times chromosomes interact during chromosomal crossover when they recombine. Chromosomal crossover is when two DNA helices break, swap a section and then rejoin.
Recombination allows chromosomes to exchange genetic information and produces new combinations of genes, which increases the efficiency of natural selection and can be important in the rapid evolution of new proteins. Genetic recombination can also be involved in DNA repair, particularly in the cell’s response to double-strand breaks.
The most common form of chromosomal crossover is homologous recombination, where the two chromosomes involved share very similar sequences. Non-homologous recombination can be damaging to cells, as it can produce chromosomal translocations and genetic abnormalities. The recombination reaction is catalyzed by enzymes known as “recombinases”, such as RAD51.
The first step in recombination is a double-stranded break either caused by an endonuclease or damage to the DNA. A series of steps catalyzed in part by the recombinase then leads to joining of the two helices by at least one Holliday junction, in which a segment of a single strand in each helix is annealed to the complementary strand in the other helix. The Holliday junction is a tetrahedral junction structure that can be moved along the pair of chromosomes, swapping one strand for another. The recombination reaction is then halted by cleavage of the junction and re-ligation of the released DNA. DNA recombination refers to the process that a DNA segment moves from one DNA molecule to another DNA molecule.
Q.6. What do you mean by RNA Isolation?
Ans. RNA is a biological macromolecule that serves a number of different functions. Messenger RNA (mRNA) transcribed from DNA, serves as a template for synthesis of proteins. RNAs are also a part of ribo-proteins involved in RNA processing.
Many viruses contain RNA as their genome. A typical mammalian cell contains 10-30 pg total RNA. The majority of RNA molecules are tRNAs and rRNAs, while mRNA accounts for only 1%-5% of the total cellular RNAs.
The actual amount depends on the cell type and physiological state of the cells. Approximately 360,000 mRNA molecules are present in a single mammalian cell, made up of approximately 12,000 different transcripts with a typical length of approximately 2 kb. While the genes of an organism are relatively fixed, the mRNA population represents how genes are expressed under any given set of conditions.
Compared to DNA, RNA is relatively unstable. This is largely due to the presence of ribo- nucleases (RNases), which break down RNA molecules. RNases are very stable, do not require co-factors, are effective in very small quantities, and are difficult to inactivate.
RNase contamination can come from human skin and dust particles, which can carry bacteria and moulds. Isolation and analysis of RNA therefore requires specialized techniques.
Since RNases are difficult to inactivate and even minute quantities can destroy RNA, proper microbiological, aseptic technique should always be used when working with RNA. Hands and dust particles may carry bacteria and moulds, and are the most common source of RNase contamination.
Always wear latex or vinyl gloves while handling reagents and RNA samples. Non-disposable plasticware should be thoroughly rinsed with 0.1 N NaOH, 1 mM EDTA followed by RNase free water.
Alternatively, chloroform-resistant plasticware can be rinsed with chloroform to inactivate RNases. Glassware should be cleaned with a detergent, thoroughly rinsed and oven dried for at least 4 hours before use.
Alternatively, glassware can be treated with DEPC (diethyl pyrocarbonate). Fill glassware with 0.1% DEPC in water and incubate 12 hours at 37°C, and then autoclave.
Q.7. How to isolate RNA from Acid Phenol?
Ans. Protocol:
1. Insert tissue or cell sample (up to 1 g) into a 15 ml polypropylene tube on ice.
2. Add 4 ml of denaturing solution (2-mercaptoethanol added fresh), and homogenize with the glass homogenizer until tissue is completely disrupted. Keep the tube on ice.
3. Add 400 μl of 2 M sodium acetate pH 4.0 and mix.
4. Add 4 ml of water-saturated phenol. Mix.
5. Add 800 μl of chloroform-isoamyl alcohol (49:1). Shake vigorously for 10 seconds.
6. Store on ice for 15 minutes.
7. Centrifuge for 20 minutes, 8000 rpm at 4°C.
8. Transfer the upper phase to a 15 ml corex tube.
9. Add 1 volume of cold isopropanol and store at -20°C for at least 1 hour.
10. Centrifuge for 20 minutes, 8000 rpm at 4°C.
11. Re-suspend pellet in 1.2 ml of denaturing solution. Add 2.4 ml cold ethanol and store for 1 hour at -20°C.
12. Centrifuge for 20 minutes, 8000 rpm at 4°C.
13. Re-suspend in 1 ml of TE (RNA grade) with proteinase K (100 μg/ml).
14. Incubate 30 minutes, 37°C.
Split into four 1.5 ml Eppendorf tubes. Extract with equal volume phenol, then phenol- chloroform (water-saturated phenol in both cases). Add 0.1 volume of 3 M sodium acetate pH 5.2 (DEPC-treated), and 2.5 volume of cold ethanol. Store tubes at -20°C until ready to use. RNA is very stable when stored in this manner.
The integrity of RNA can be checked rapidly by running 1 μl on an RNase-free agarose gel (clean the box well in SDS) and staining with ethidium bromide. Strong 28S and 18S bands should be visible.
Preparation of Solutions:
To dissolve Sarcosyl, warm to 65°C.
Do not add the 2-mercaptoethanol until just before use.
When ready, add 7.2 μl 2-mercaptoethanol per ml of solution.
2M Sodium Acetate pH 4.0:
Dissolve 16.4 g in 80 ml distilled water.
Adjust the pH to 4.0 with glacial acetic acid
Make up the volume to 100 ml.
Water Saturated Phenol:
Thaw an appropriate amount of high grade (or re-distilled) phenol at 65°C.
Add an equal volume of water (DEPC-treated), shake 30 seconds, and centrifuge at 4000 rpm for 10 minutes.
Remove the upper (aqueous) phase, and preserve the lower (phenol) phase.
Q.8. What are the major steps of cloning?
Ans. There are two major steps available for the introduction of plasmid DNA into bacterial cells.
They are:
i. Preparation of competent cells,
ii. Transformation of the competent cells,
The key development as far as transformation is concerned, occurred in the early 1970s, when it was demonstrated that E. coli cells soaked in an ice-cold salt solution were more efficient in DNA uptake than un-soaked cells.
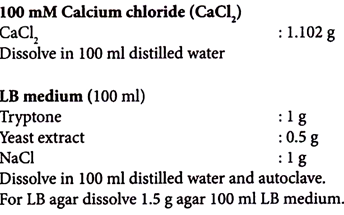
It is possible to cause the DNA to precipitate on the outside of the cells using calcium chloride; perhaps the salt is responsible for some kind of change in the cell wall that improves DNA binding. Soaking the cells in calcium chloride affects only the DNA binding and not the actual uptake into the cells.
When DNA is added to the treated cells, the DNA remains attached to the cells exterior. The actual movement of DNA into the competent cells is stimulated briefly by raising the temperature to 42°C.
It is also said transformation is induced by destabilization of the lipids in the cell envelope through heat shock treatment. Various other factors also play a vital role in transformation like plasmid size, DNA configuration (closed, linear, nicked) and antibiotic or marker selection.
i. Preparation of Competent E. coli Cells:
Cells that have the ability to take up DNA from various sources are termed ‘competent’.
1. Pick a single colony of E. coli from the freshly streaked plate and inoculate into 2 ml of LB medium.
2. Grow overnight at 37°C in a shaker.
3. Inoculate 0.5 ml of the overnight culture into 50 ml of LB medium and leave it in a shaker at 37°C until an OD of 0.4 to 0.5 at 600 nm is achieved (approximately 90-120 minutes). Keep the culture on ice.
4. Centrifuge the culture at 5000 rpm for 10 minutes at 4°C. Collect the cells and keep the cells on ice.
5. Suspend the cells gently in 25 ml of 100 mM ice-cold CaCl2 solution.
6. Centrifuge at 5000 rpm for 10 minutes at 4°C. Collect the pellet.
7. Re-suspend the pellet with 2.5 ml of pre-cooled 100 mM CaCl2 and leave it on ice for further 30 minutes.
(Prepare aliquots of 100-200 µl in sterile micro-centrifuge tubes and freeze in liquid nitrogen or a dry ice-ethanol mix. Store the competent cells at -70°C).
ii. Transformation of Competent E. coli Cells:
Transformation is the process by which plasmid DNA is introduced into a bacterial host cell.
1. Transfer an aliquot (10 µl) of the DNA (cloned plasmid) to be transformed into a cold sterile micro-centrifuge tube, and keep it on ice.
2. Transfer gently 100 µl of cell suspension (competent cells) into the micro-centrifuge tube, mix carefully and keep it on ice for 20 minutes.
3. Transfer the tube to a water bath and incubate for 90 seconds at 37°C.
4. Add 500 µl of LB medium to the cells and incubate for 60-90 minutes at 37°C.
5. Plate using 50, 100 and 200 pi aliquots on LB-agar plates containing relevant antibiotic (marker).
6. Incubate the plates overnight at 37°C until colonies develop.
Transform competent cells with 1 ng of a ‘control plasmid’ containing an antibiotic resistance gene. Plate onto LB-agar plates containing relevant antibiotic. Compare the number of colonies obtained with the control plasmid to the number obtained with the ‘plasmid of interest’ (cloned plasmid) to compare transformation efficiency
Keep a ‘negative control’ (cells without plasmid) and plate these cells on an agar plate containing the relevant antibiotic. Absence of colonies on the plates indicates that the antibiotic is active.
Q.9. What do you understand by the term ‘Restriction Enzyme Digestion of DNA’?
Ans. Restriction endonucleases are bacterial enzymes that bind and cleave DNA at specific target sequences. Type II restriction endonucleases are the most widely used in molecular biology applications.
These enzymes bind DNA at specific recognition site, consisting of a short palindromic sequence, and cleave within this site. Restriction endonucleases with shorter recognition sequences cut more frequently than those with larger recognition sequences. For example a 4 base pair cutter will cleave, on average, every 44 (256) bases, while a 6 base pair cutter cleaves every 46 (4096) bases.
Use a 6 bp cutter for mapping genomic DNA, as these give fragments of a size range suitable for cloning. Some enzymes cut both DNA strand at the same position, creating blunt-ended DNA fragments. But, majority of enzymes make cuts, which form sticky ended DNA fragments.
Some enzymes create 5′ overhangs and other creates 3′ overhangs. Sticky-ended DNA fragments can be easily ligated to other sticky ended overhangs, resulting in efficient cloning. Blunt ended DNA fragments usually ligate less efficiently, making cloning more difficult.
The genomic DNA of some organisms is methylated at specific sequences (for example, the dinucleotide sequence CpG). Methylation patterns differ in different species, affecting the choice of restriction enzymes.
The restriction enzymes with a CpG dinucleotide in their recognition site do not cleave if the cytosine is methylated. Therefore many enzymes with CpG in their recognition site, such as Eag I, Not I and Sal I, do not cleave DNA.
One unit of restriction endonuclease completely digests 1 μg of substrate DNA in 1 hour. However, genomic DNA generally requires more than one unit of enzyme per μg of DNA for complete digestion.
Many times a 10-fold excess of enzymes is added to the reaction mixture in order to ensure complete digestion. Ensure that the enzyme volume does not exceed more than 10% of the total reaction volume, as otherwise the glycerol in which the enzyme is supplied may inhibit digestion.
1. Add the following to a microfuge tube –
10X Restriction Endonuclease buffer – 2 μl (1 X final concentration)
DNA – 2 μl (0.1 to 5 μg)
Distilled water – 15 μl
Restriction Endonuclease (enzyme) – 1 μl (3 to 20 units)
2. Gently mix the contents after the addition of enzyme.
(These volumes are for analytical digests. Larger volumes may be needed for preparative digests and chromosomal DNA digests).
3. Centrifuge for a few seconds in microfuge.
4. Incubate at 37°C in a water bath or dry bath or oven usually for 1-4 hours.
(Restriction enzymes isolated from thermophilic bacteria require higher incubation temperatures, for example – 50-65°C.)
5. Heat inactivate the enzyme after digestion by heating the reaction to 65°C for 20 minutes after digestion (majority of enzymes that have an optimal incubation temperature of 37°C are inactivated).
6. Run the digests on a gel to check for digestion.
Q.10. What is the role of Agarose Gel Electrophoresis in DNA:
Ans. Agarose gel electrophoresis allows separation, identification and purification of nucleic acids based on charge migration. Migration of nucleic acid molecules in an electric field is determined by size and conformation, allowing separation of different size nucleic acid fragments.
However, the relationship between fragment size and rate of migration is non-linear, since larger DNA fragments have greater frictional drag and are less efficient at migrating through the polymer.
This type of gel electrophoresis is used to analyze DNA fragments between 0.1 to 25 kb. Analysis of larger fragments up to 10,000 kb is possible with the help of pulse field gel electrophoresis.
Agarose is a polysaccharide extracted from seaweed. It is typically used at concentrations of 0.5 to 2%. Increased agarose concentration makes the gel stiffen Agarose gels are extremely easy to prepare.
It is also non-toxic. By varying the concentrations of agarose, fragments of DNA from about 200 to 25,000 bp can be separated using standard electrophoresis techniques. The concentration of agarose used for the gel depends on the size of the DNA fragments to be analyzed.
Low agarose concentrations are used to separate larger DNA fragments and high agarose concentrations are used to resolve smaller DNA fragments. Ultra-pure agarose usage is largely used since impurities in the agarose such as polysaccharides, salts and proteins can affect the migration of DNA. The quality of agarose is most important when running high percentage agarose gels.
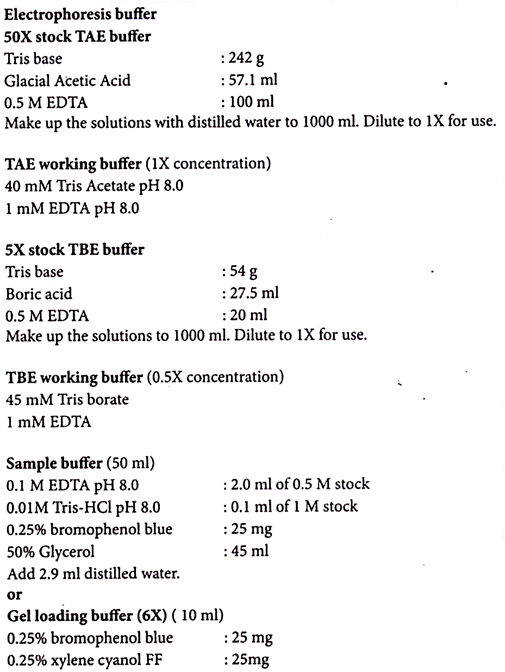
Several different buffers are recommended for electrophoresis of DNA. The most commonly used for double stranded DNA fragments are TAE (Tris-acetate-EDTA) and TBE (Tris-borate-EDTA).
DNA fragments will migrate at different rates in these two buffers due to differences in the ionic strength. Buffers not only establish pH, but also provide ions to support conductivity.
TAE has lower buffering capacity than TBE and is more easily exhausted during extended electrophoresis. TBE should be used for pulse-field electrophoresis due to high voltages used in this procedure.
1. Prepare 1X electrophoresis buffer for both gel and electrophoresis.
2. Weigh an appropriate amount of agarose to the appropriate volume of 1X electrophoresis buffer in a conical flask (the flask should not be more than half full and covered to minimize evaporation).
3. Heat in a microwave or boiling water bath, rotate the flask occasionally, until the agarose is dissolved.
(If the volume reduces during heat due to evaporation, makeup the original volume with distilled water. This will ensure that the agarose concentration is maintained.)
4. Cool the agarose to 55-60°C using running tap water.
5. Insert the comb before pouring the gel on to the gel tray.
(Ensure that there is enough space between the bottom of the comb and the gel tray, about 0.5 to 1.0 mm to allow proper formation of wells and avoid sample leakage.)
6. Pour the agarose solution on to the gel tray (Seal the open edges of the tray provided with the apparatus with the help of cellophane tape before pouring the agarose.)
7. Leave the gel to solidify for 30-40 minutes.
(Make sure that there are no air bubbles in the gel or in the well.)
8. Carefully remove the comb and the cellophane tape from the gel tray.
9. Fill the electrophoresis tank containing the gel with 1X electrophoresis buffer.
(Add enough buffer to cover the gel with 1 mm liquid above the surface of the gel. If too much buffer is used the electric current will pass through the buffer instead of the gel.)
10. Add 1 volume of sample buffer to 5 volumes of DNA sample and mix.
11. Apply samples to the wells formed in the gel.
(Prior to sample loading remove any air bubbles from the wells and rinse with electrophoresis buffer; insert pipette tip into the well to load samples and expel the sample slowly; after sample loading do not move the electrophoresis tank or tray as this may cause samples to float out of the wells; include always a lane for appropriate molecular weight marker.)
12. Connect the electrodes and apply the current (1-10 volts/cm of the gel) until the dye has migrated an appropriate distance in the gel.
(Avoid the use of high voltages, which can cause trailing and smear of DNA particularly with high molecular weight DNA. Melting of agarose during electrophoresis indicates incorrect preparation of the gel or exhausted ions in the buffer during the run. Recycle the buffer using a pump for very long runs.)
13. After electrophoresis stain the gel with ethidium bromide to visualize the DNA bands. (This commonly used dye is intercalating and fluorescent in nature, and can be either used before or after electrophoresis.
Stock solutions of this stain (10 mg/ml) should be stored in dark bottles or wrapped in aluminium foil. Ethidium bromide at a concentration of 0.5 μg/ml is also normally incorporated in the gel before electrophoresis.
The gel is soaked otherwise in electrophoresis buffer or water containing 0.5 μg/ml ethidium bromide for 30-40 minutes. The gel is rinsed with buffer or water before analyzing it to remove excess ethidium bromide).
14. Visualize the gel under UV light (254-366 nm).
(This increases florescence of ethidium bromide and DNA complexes. UV light damages the DNA and if DNA fragments are to be extracted from the gel, use a lower intensity UV source and minimize the exposure of the DNA to UV light. UV light can damage the eyes and skin. Wear suitable eye and face protection when working with UV light.)
Q.11. What is the role of Agarose Gel Electrophoresis in RNA?
Ans. Formaldehyde agarose gels allow separation and identification of RNA based on charge migration. Unlike DNA, RNA has a high degree of secondary structure, making it necessary to use a denaturing gel. Formaldehyde in the gel disrupts the secondary structure so that RNA molecules can be separated by their charge migration.
Nucleic acid molecules in an electric field migrate towards anode due to their negatively charged phosphates along the backbone. The migration of denatured molecules is determined by their size.
Small RNA molecules such as tRNAs or 5S rRNAs can be analyzed using polyacrylamide gels. An agarose concentration of 1.0% to 1.2% (w/v) is used for most RNAs. For larger mRNA species, it may be helpful to reduce the agarose concentration and for smaller mRNAs the agarose concentration can be raised to 2%.
1. Mix appropriate amount of agarose in 10X formaldehyde agarose gel buffer.
2. Heat the mixture in a microwave or boiling water bath, rotating the flask occasionally until agarose is dissolved.
3. Cool the agarose to 65-70°C in a water bath. Mix to prevent uneven cooling.
4. On cooling add 1.8 ml of 37% formaldehyde (12.3 M) (for 100 ml gel buffer).
(Make sure that the solution has cooled sufficiently before adding formaldehyde. Formaldehyde is volatile and it may evaporate if added to the hot solution.)
5. Pour the agarose onto the gel tray (in a fume hood) to a thickness of 3-5 mm.
(Thicker gels can be used to load more samples. Thinner gels are always better for northern blotting.)
6. Insert the comb immediately before adding the agarose.
(Thinner combs give sharper bands and thicker combs allow more samples loading.)
7. Allow the gel to polymerize for 30 minutes.
8. Place the gel in the electrophoresis tank and fill it with IX formaldehyde agarose electrophoresis buffer.
9. Carefully remove the comb from the gel. Pre-run the gel in IX formaldehyde agarose electrophoresis buffer for 30 minutes.
10. Add 1 volume of RNA sample loading buffer to 5 volumes of RNA sample and mix.
11. Incubate the mixture at 65°C for 3-5 minutes to denature the RNA and chill on ice.
12. Apply samples to the wells of the gel.
13. Run the samples (at 5-7 volts/cm) until the bromophenol blue has migrated approximately 2/3 of the way through the gel.
14. After electrophoresis stain the gel with ethidium bromide as described for DNA gel and illuminate the stained gel under UV at 254-366 nm.
RNA agarose gels are run at lower pH than DNA gels since RNA has a lower pKa value than DNA. Further RNA is susceptible to alkali cleavage at higher pH. MOPS (3-N-morpholinopro- panesulfonic acid) is the most commonly used for RNA buffers due to its high buffering capacity at pH 7.0. Formaldehyde is included in the running buffer to keep the RNA denatured.
RNA loading buffer serves the following purposes:
1. Formaldehyde to denature the RNA sample prior to loading
2. Glycerol to increase the density of the sample
3. Bromophenol blue as marker dye.
Q.12. What is 2D Gel Electrophoresis (2D PAGE)?
Ans. Proteomics is a methodology that is used to identify and characterize the protein complement of the genome. It is the descriptive classification of cell or tissue protein expression for physical, functional and interactive mapping of proteins within subcellular compartments.
Although sophisticated technology has developed in the field of proteomics, the technique which is considered as the oldest—two-dimensional polyacrylamide gel electrophoresis (2D PAGE) followed by peptide mass fingerprinting (PMF and MALDI-MS TOF)—possibly remains unmatched for its combination of resolving power, quantitative information, indication of post-translational modification, accessibility, and its relatively high-throughput efficiency.
Two-dimensional gel electrophoresis (2D PAGE) is a method of protein separation by which proteins in a mixture are separated first according to their isoelectric point (pI), called isoelectric focusing (IEF), and then on the basis of their molecular weight in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
The 2D PAGE is used for the isolation, separation and purification of proteins for further characterization with the mass spectrometry and identification of specific proteins. The isoforms of a protein can easily be isolated with 2D PAGE.
1. Stain the IEF gel using regular Coomassie brilliant blue and destain.
2. Cut out the desired lane (strip) from the gel for transfer to a SDS gel (if IEF is performed in slab gel. Alternatively, commercially available narrow pi range IPG strips can also be used).
3. Incubate the gel strip in 2 ml of 2X SDS sample buffer and 0.5 ml of ethanol for 3-5 minutes. Aspirate the sample buffer and rinse the gel strip with 1X SDS running buffer. This step loads the sample with SDS and reduces alkylate sulfhydryl moieties.
4. Fill the SDS gel cassette with 1X SDS running buffer.
5. Trim the IEF strip to a length that will accommodate the gel cassette. Transfer the gel strip by sliding the strip onto the gel cassette and into the 2D-well using a gel-loading tip without forming any air bubbles.
6. Cut a piece of thick filter paper to the length of the IEF strip and wet in SDS running buffer; insert the long edge of the paper into the SDS gel. Push the filter paper down so the gel strip makes contact with the SDS gel.
7. Prepare a sandwich of the filter papers and gel strip along the long edge of the paper, such that the gel strip is sandwiched between the two filter papers.
8. Insert gel cassette into the electrophoresis unit, fill the buffer chambers with 1X SDS running buffer, and perform SDS-PAGE.
9. After the dye front has moved into the stacking gel (approximately 10 minutes), disconnect power, remove the filter papers, and resume electrophoresis.
10. Refer to the SDS-PAGE protocol for electrophoresis and sample staining.