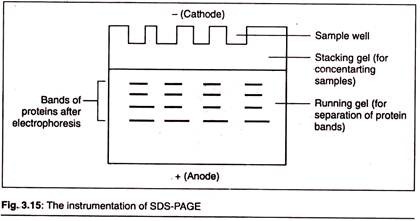
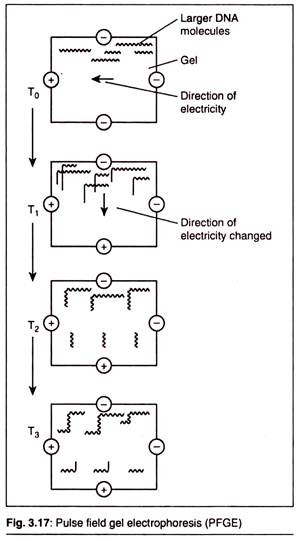
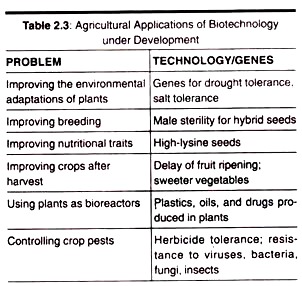
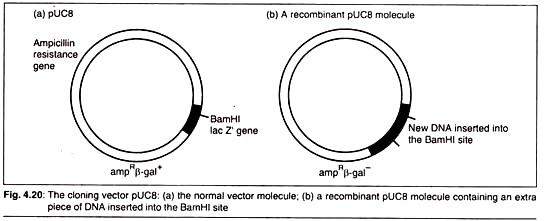
Here is a list of microbial polysaccharides:- 1. Xanthan 2. Pullulan 3. Dextran 4. Cyclodextrins 5. Gellan 6. Welan 7. Curdlan 8. Polyhydroxybutyrate.
1. Xanthan:
Xanthan (or, as commonly called, ‘xanthan gum’) at $14/kg falls into a similar price range to other gums and exudates, has similar functional properties. It is made by the organism Xanthomonas campestris (which in its wild existence is responsible for cabbage blight), grown largely on glucose, itself derived from maize starch. It converts glucose with high efficiency (80 percent) to the xanthan gum. Typically for a fermentation product, the raw material costs are a small part of the total, which are mainly due to recovering the gum from the culture medium.
Glc = Glucose, Mann = Mannose, GluA = Glucuronic acid,
MannA = Mannuronic acid, Ac = Acetate
Production is around 9000 tonnes a year. It has a β (1 → 4) linked glucan main chain with alternating residues substituted on the 3-position with a trisaccharide chain containing two mannose and one glucuronic acid residue. It is thus a charged polymer. Some of the mannose residues may also carry acetyl groups.
It is useful because it forms relatively rigid-rod-like structures in solution at ambient temperature, though they convert to the random configuration on heating. These rods are able to align themselves like agarose and the k- and t- carrageenans – with the unsubstituted regions of galactomannans, such as guar and its derivatives and locust bean gum, to produce fairly rigid mixed gels with applications in food manufacture.
The primary structure (as worked out by sequential degradation and methylation analysis) consists of the same backbone as cellulose:
…→ 4) β-D-Gicp (1 → …
with the substitution at C3 of every alternate GIcp residue by the negatively charged trisaccharide
β-D-Manp-(1 → 4) β-D-GicpA-(1 → 2) α-D-Manp-6-O-acetyl (1 → 3)
with some of the terminal β-D-Manp residues substituted at positions C4 and C6 by pyruvate (CH3.CO.COO–). Thus xanthan (expect under highly acidic conditions) is a highly charged polyanion. Figure 10.1 shows the Haworth projection of a (pyruvylated) alternate repeat section of xanthan.
The biosynthetic pathway of xanthan is depicted in Fig. 10.2.
2. Pullulan:
Pullulan is a glucan made up from maltotriose units linked by 1,6 bonds ( → 6)- α–D-Glc- (1 → 4)- α -D- Glc-(1 → 4)- αD-Glc-(1 →). It is obtained from Aureobasidium pullulans and is hydrolyzed by pullulanase to yield maltotriose. It is not attacked by digestive enzymes of the human gut, and is used to form films. Production is now substantial, and has found particular application in formulating snack foods in Japan based on cod roe, powdered cheese and as a packaging film for ham.
It is water soluble, with molecular weights in the range of 5000 Da to 900000 Da, with straight unbranched chains, behaves as a random coil according to a combination of sedimentation coefficients and intrinsic viscosity measurements. Similarly, light scattering and sedimentation equilibrium measurements show that pullulans above about 50000 Da molecular weight behave to a close approximation like a classical random coil.
It has been proposed as a ‘standard polysaccharide’ in the sense that it is so near to random coil behaviour and readily obtainable in very reproducible form that it could be used for comparative tests with other polysaccharides. The idea is presumably that deviation from pullulan-type behaviour must imply a more complex structure. It might also be used to standardize instruments. The solution in water are liable to bacterial attack with rapid degradation of the molecular weight and preservatives such as azide may be needed.
Pullulan gel is heat stable and remain unaffected by pH and compatible with most electrolytes. Barium and titanium increases viscosity dramatically. Its viscosity is not changed in presence of NaCl and 100°C. It is highly biodegradable and soluble in water. Properties of pullulan can be changed by esterification, etherification or cross linking.
It is first commercially produced in 1976 by Hayastihara chemical co. It is produced by A. pullulans in 15% acid hydrolysed starch, 0.2% K2HPO4, 0.2% NaCl, 0.2% Peptone, 0.04% FeSO4, pH 6.5, incubation temperature 30°C aerobic, incubation period 100-125 hrs. It is diluted with water and precipitated with isopropane.
Applications:
It is extensively used in food, pharmaceutical and industrial sector. It is considered to be safe and non-toxic product. It is a good substitute for low caloric additive to wheat flour or starch in bakery products and dietary foods. It is used as a thickener in beverage, creams, frosting, fillings, sauces, dried vegetables and meats.
Package with this polysaccharide helps to retain freshness and prevent oxidation over long periods. It is used in tablet coatings, contact lenses, pharma expanders. Pullulan coatings are for lithographic printing and plate protection. It acts as a binder for the preparation of tobacco sheets. Acts as a boundary sand binder.
3. Dextran:
Bacterial dextrans are produced in substantial quantities by Leuconostoc mesenteroides and are familiar to laboratory workers as the basis for cross-linked dextran beads used in gel filtration columns. They are mostly α (1 → 6) D-glucopyranosyl polymers with molecular weights up to = 1 × 106 Da more or less branched through (1 → 2), (1 → 3) or (1 → 4) links (Fig. 10.4).
In most cases the length of the side chains is short, and branched residue vary between 5 and 33 per cent. The major commercial dextran is about 95 per cent (1 → 6) linked, 5 per cent (1 → 3) linked, and is made by selected strains of Leuconostoc. After ethanol precipitation from the culture medium acid hydrolysis is used to reduce the overall molecular weight, though fungal dextranases can also be used.
Sequential degradation of dextran established that 40% of side chain is one unit long, 45% are two units long, 15% contain more than two units and overall 5% degree of branching molecular weight of native dextran ranges from 2 × 106 to 5 × 106 daltons.
The product with an average molecular weight of about 60000 Da is used in medicine as a blood extender, while fractions of defined molecular weights (e.g. the Pharmacia ‘T’-series, where ‘T500 Dextran’ would stand for dextran of weight average molecular weight 500 kDa) are familiar in laboratories and to some extent, like the pullulan ‘P’-series, serve as polysaccharide standards in molecular weight calibrations. They are also used as part of incompatible phase separation systems, usually with polyethylene glycol. ‘Blue dextran’ is a well-known marker for the void volume for gel filtration studies.
Dextran is produced in a medium containing 2% sucrose which induces dextran sucrose, vitamin cofactors, amino acid supplements as yeast extract, corn steep liquor, acid hydrolysed casein, malt extract, peptone, tryptone broth, phosphate in 0.1 – 0.5% and pH 6.7 – 7.2, incubation temperature is 25°C. Dextrans have been found very wide applications in laboratory work because they are particularly free from positive interactions with proteins.
The interaction can be almost entirely characterized as coexclusion. This has been found application, as already noted, in gel filtration media, but cross-linked dextran gels show other effects. For example – they swell and shrink in a way related to the osmotic pressure of the solvent system and can be used to make miniature osmometers. Dextran 70 is used for treatment of shock reading risks thrombiasis, post-operative pulmonary emboli. Dextran 40 is used in reduction of blood viscosity and erythrocyte agglutination.
4. Cyclodextrins:
Cyclodextrins have attracted more interest than any other bacterial polysaccharide because of their unique structures. The interest has been more scientific than industrial, since xanthans are much more important in commercial terms, but there always seems to be a potential application of the cyclodextrins on the horizon which would lead to a major expansion in demand for them.
Formerly called Schardinger dextrins after their discoverer’s still called cyclomyloses name cyclomaltoses here and there, they are cyclic molecules formed by glucopyranose linked by α-D-(1 → 4) linked bonds. They contain six, seven, eight or nine glucopyranose residue. It is impossible to form cyclic structures with less than six residues, while those with more than nine have so far been found to have at most a nine-membered ring with other glucose residues attached as branches through (1 → 6) links. The structures (for a seven-membered ring, cGIc7 or in common notation cGIu7) are illustrated in Fig. 10.5.
The nine-membered ring is uncommon and found only as a minor by-product of enzymatic synthesis of the other. The six, seven and eight-membered rings are sometimes called as α-, β-, and γ -cyclodextrins, abbreviated as cGIu6, cGIu7, cGIu8.
They are water soluble, easily crystallized and their structures have been determined. The great interest in them is because the cavity is of a size to include many small molecules of interest and is also fairly hydrophobic in character. The outside of the toroidal cone is full of hydrophilic hydroxyl groups.
As might be expected, most of the potential applications involve inserting small molecules into the cavity. The dimensions are roughly those of a cylinder with length of 7 Å, diameter of 4.5 Å for the six-membered ring and 8.5 Å for the eight-residue ring. Thus they can accommodate an aromatic ring like benzene, as well as alkyl chains.
Applications:
1 cyclodextrins are competitive insulators of α-and β-amylases, potato phosphoxylase, pullulanase are used as low calorie.
5. Gellan:
Gellan is an extracellular polysaccharide produced by Pseudomonas elodea. Gellan consist of a linear tetrasaccharide sequence containing two D-glucopyranosyl units, one D-glucano pyranosyl unit and one Rhamnopyranosyl unit plus the acyl groups, acetyl, L-glyceryl units attached to 0- 6,0-2 respectively of one of the D-glucopyranosyl units (Fig. 10.6).
This glucose carries 0-acetyl and glyceryl residues in the native polymer. The molecular weight of gellan is estimated to be 1 or 2 × 106 daltons. The polysaccharide is characterized as a parallel, half staggered double helix in which each polymer chain is in a left-handed, 3-fold helical confirmation and in which two such duplexes are packed antiparallel to each other in the unit cell. It is sensitive to calcium levels but has rheological properties similar to those of xanthan which has a similar charge density.
It was clearly intended to be a xanthan competitor though before permission for food use was obtained it was promoted as an agar substitute particularly for use in growth media. It is produced in submerged aerobic fermentation. The medium consists of D-glucose, NH4NO3, soy protein hydrolysate, KH2PO4 and trace element solution. The viscosity of medium reaches 5000-6000 centipolses. Gellan is recovered from broth by isopropanol precipitation which is dried, milled and packed.
(i) Properties:
Gellan by deacylation produces firm, brittle gels. It shows thermo reversible viscosity changes. Cooling with ion free water can be heated and cooled without gelation. It has a good thermal stability and can be autoclaved without loss of viscosity. It requires either monovalent or divalent cations for gel formation. Texture and strength of gel can be changed with the anion and its concentration.
(ii) Applications:
It can serve as a substitute for agar in preparation of solid media for microorganisms. It is used in plant tissue culture experiments. Its requirement is 1/4th to that of agar. It is used in antigen-antibody diffusion technique and as a matrix for enzyme and cell immobilization.
6. Welan:
Welan commercially known as Biozan. It is similar to gellan having single side chain unit. It has high thermostability (at 135°C/hr). It is produced by Acaligenes sp. It is produced under submerged aerobic fermentation. The medium composed of glucose, soy protein, hydrolysate, phosphate buffer, NH4NO3 and trace elements is used for production of welan. Extracellular polysaccharide is recovered by precipitation with isopropyl alcohol.
Applications:
It is used in drilling fluid additive. It is used in oil recovery.
7. Curdlan:
Curdlan, α β (1-3) linked D-glucan discovered in 1996, is a thermogelable polysaccharide (1 → 3) – β -D Glc-(1→. This glucose carries residues in the native polymer. It is produced by Alcaligenes faecalis v. myxogenes in a medium containing 4% glucose, 0.1% citrate, succinate or fumarate, 0.55% (NH4) HPO4 and mineral salts. It is insoluble in acidic and neutral water but soluble in alkaline water. It is soluble in formic acid, dimethyl sulfoxide, saturated solution of urea or thiourea or 2% potassium.
At 55°C it becomes clear solution. Curdlan gels are stable over wide pH range and stable to freeze thaw. It forms a firm resistance high set gels that melts at 140 + 160°C. Its annual production exceeds 200-300 tons. It is nutritionally inert and non-toxic. Mutants of Agrobacterium which produces curdlan in pure form and in high yields, is being used in Japan for commercial production.
Applications:
It helps to improve texture of different foods such as tofu, yokan, boiled fish paste, noodles, sausage, jellies and jams. It masks odors, prevents scording aroma and adhesion. Curdlan is a good binder in animal feed, carrier for immobilized enzymes and substitute soil for rice plants.
8. Polyhydroxybutyrate:
Polyhydroxy alkanoates (PHAs) have considerable potential as biogradable alternatives to petroleum derived plastic PHAs are linear homochiral thermoplastic polysters produced as intra cellular energy reserves by numerous microorganisms. The most widely encountered PHAs are poly β hydroxybutyrate (PHB) and polylactic acid (poly hydroxy propionate) formed from the monomers of hydroxybutytic acid and lactic acid respectively.
Bacteria differ in their food reserve with respect to genotype of the organism and nutrient limitation if the carbon to nitrogen ratio is high and if nitrogen or oxygen is limiting. Many bacteria accumulate glycogen/aliphatic polyester (poly 3-hydroxyalkanoates) in amounts ranging from 30-80% of their cellular dry weight. Polyhydorxy alkalnoates produced from naturally occurring organic substrates have the general structure (Fig. 10.7).
General formula is to be given:
Where α = 0-8 or higher. The asterisk indicates an asymmetric centre, α-cmoigne (1926) for the first time recorded poly-(RR) (hydroxybutryate) (PHB) in Bacilus megaterium. It was only after many years it came to know this thermoplastic material which melts when heated to certain temperature but harden again as they cool. This cycle can be repeated many times. This in contrast to familiar thermoplastic material such as polyethylene, polypropylene, polystyrene, polycarbonate and neston which are non-biodegradable are readily biodegradable. Some of the differences between two materials are listed table 10.4.
The difference in density of two materials makes polypropylene to float on water while poly (3-hydroxybutryate) sink to the bottom which facilitates to degradation. Further biodegradable polyhydroxy butyrate has many advantages in the manufacture of packing containers, bottles, wrapping films, bags and the like.
They have important medical uses such as sensing as surgical pans, staples wound dressings, bone replacements, plates and biodegradable carriers for the long-term release of medicine derivates of polylactic acid which have been used in medicine as template for tissue growth and in plastics for replacement forms. It is also used in food storage materials and shampoo bottles.
Polyhydroxy alkanoates (PHAs) have considerable potential as biodegradable alternatives to petroleum derived non-biodegrade plastics such as poly propylene, ethyleneglycol. These are linear homochiral thermoplastic polyesters produced as intra cellular energy reserves by numerous microorganims. These biopolymers accumulate as distinct 0.2-0.7 μm diameter granular inclusion bodies in response to nutrient limitation especially in pseudomonads (Fig. 10.7).
The most widely encountered PHAs are polyhydroxy butyrate (PHB) and polylactic acid (polyhydroxy propionate) formed from the monomers hydroxybutyric acid and lactic acid respectively.
The biosynthetic pathway of PHB Arabidopsis thaliana is depicted in Fig. 10.8.
First is the biosynthesis of the hydroxyacyl CoA monomers followed by their head to tail polymerization to form the polymer chain which exceeds 10,000 units in length. The most fully characterized pathway is that of PHB biosynthesis in Ralstonia eutropha (= Acaligenes eutropus) Fig. 10.9.
This involves three enzymes thiase catalyses a claisen condensation of two molecules of acetyl CoA to form acetoacetyl CoA which is reduced to the chiral intermediate R-3-hydroxybutyl CoA by a reductase. Polymerization is then performed by a PHA synthetase. PHAs are synthesized at stationary phase where nutrient exhaustion is a common feature and the granules get accumulated.
In view of its high cost, efforts made to cheaper means of production are being sought. PHAs are now synthesized by recombinant microorganisms’ i.e. E. coli which contain the genes encoding the enzymes necessary for PHA biosynthesis. Such microbial recombinants may become an economically attractive source of PHAs. Alternatively transformation of a hygen plant in these genes provide an even cheaper means of PHA production in the longer term likely transgenic hosts which include Arabideopsis thaliana and Brasssica napvs or Zea mays.