The following points highlight the eight main types of fermentations. The types are:- 1. Batch Fermentation 2. Continuous Fermentation 3. Fed Batch Fermentation 4. Anaerobic Fermentation 5. Aerobic Fermentation 6. Surface Fermentations 7. Submerged Fermentations 8. State Fermentation.
Type # 1. Batch Fermentation:
A batch fermentation is a closed culture system, because initial and limited amount of sterilized nutrient medium is introduced into the fermenter. The medium is inoculated with a suitable microorganism and incubated for a definite period for fermentation to proceed under optimal physiological conditions. Oxygen in the form of air, an antifoam agent and acid or base, to control the pH, are being added during the course of fermentation process (Fig. 2.11).
During the course of incubation, the cells of the microorganism undergo multiplication and pass through different phases of growth and metabolism due to which there will be change in the composition of culture medium, the biomass and metabolites. The fermentation is run for a definite period or until the nutrients are exhausted. The culture broth is harvested and the product is separated.
Batch fermentation may be used to produce biomass, primary metabolites and secondary metabolites under cultural conditions supporting the fastest growth rate and maximum growth would be used for biomass production. The exponential phase of growth should be prolonged to get optimum yield of primary metabolite, while it should be reduced to get optimum yield of secondary metabolites.
The used medium along with cells of microorganism and the product is drawn out from the fermenter. When the desired product is formed in optimum quantities, the product is separated from the microorganism and purified later on.
It has both advantages and disadvantages which are detailed below:
(i) Merits:
(a) The possibility of contamination and mutation is very less.
(b) Simplicity of operation and reduced risk of contamination.
(ii) Demerits:
(a) For every fermentation process, the fermenter and other equipment are to be cleaned and sterilized.
(b) Only fraction of each batch fermentation cycle is productive.
(c) It is useful in fermentation with high yield per unit substratum and cultures that can tolerate initial high substrate concentration.
(d) It can be run in repeated mode with small portion of the previous batch left in the fermenter for inoculum.
(e) Use of fermenter is increased by eliminating turn round time or down time.
(f) Running costs are greater for preparing and maintaining stock cultures.
(g) Increased, frequency of sterilization may also cause greater stress on instrumentation and probes.
(h) Fresh sterilized medium and pure culture are to be made for every fermentation process.
(i) Yield of the desired product may also vary.
(j) There will be a non-productive period of shutdown between one batch productive fermentation to the other,
(k) More personal are required.
Type # 2. Continuous Fermentation:
It is a closed system of fermentation, run for indefinite period. In this method, fresh nutrient medium is added continuously or intermittently to the fermenter and equivalent amount of used medium with microorganisms is withdrawn continuously or intermittently for the recovery of cells or fermentation products (Fig. 2.12).
Fig 2.12: Continuous fermenter
As a result, volume of the medium and concentration of nutrients at optimum level are being maintained. This has been operated in an automatic manner. The continuous fermenter has its maximum use that take long time to reach high productivity, reduces down time and lowers the operating costs.
In continuous mode, starting medium and inoculum are added to the fermenter. After the culture is grown the fermenter is fed with nutrients and broth is withdrawn at the same rate maintaining a constant volume of broth in the fermenter. In continuous mode with cell cycle, the cell mass is returned to the fermenter using micro filtrations with bacteria or screens with fungal mycelium.
A continuous fermentation is generally carried out in the following ways:
(a) Single stage fermentation
(b) Recycle fermentation
(c) Multiple stage fermentation
(a) Single Stage Fermentation:
In this process, a single fermenter is inoculated and the nutrient medium and culture are kept in continuous operation by balancing the input and output of nutrient medium and harvested culture, respectively.
(b) Recycle Fermentation:
In this method, a portion of the medium is withdrawn and added to the culture vessel. Thus, the culture is recycled to the fermentation vessel. This method is generally adopted in the hydrocarbon fermentation process. The recycling of cells provides a higher population of cells in the fermenter which results in greater productivity of the desired product.
(c) Multiple Stage Fermentation:
In this process, two or more fermenters are employed simultaneously and the fermentation is operated in a sequence. Different phases of fermentation process like growth phase and synthetic phase are carried out in different fermenters. Generally, growth phase is allowed in the first fermenter, synthetic phase in the second and subsequent fermenters.
This process is adapted particularly to those fermentations in which growth and synthetic activities of the microorganisms are not simultaneous. Synthesis is not growth related but occurs when cell multiplication rate has slowed down.
The process of continuous fermentation is monitored either by microbial growth activity or by product formation and these methods are called:
(i) Turbidostat method, and
(ii) Chemostat method.
(i) Turbidostat Method:
In this method the total cell content is kept constant by measuring the culture turbidity at a regular interval of fermentation process. By turbidity measurement it is possible to the fermenter to regulate both the nutrient feed rate and the culture withdrawal rate.
Fermentation, in which this method is employed, must be carried out at a low maximum cell population which leads to the usage of less amount of substrate and wastage of greater amount of substrate as unused and residual medium, which is removed from the fermenter along with the harvested culture (Fig. 2.13).
(ii) Chemostat Method:
In this method nutrient feed rate and harvest culture withdrawal rate are maintained at constant value. This is achieved by controlling the growth rate of the microorganism by adjusting the concentration of any one of the chemicals of the medium, like carbon source, nitrogen source, salts, O2 etc. which acts as a growth limiting factor.
Apart from the above chemicals, sometimes the concentration of the toxic product generated in the fermentation process, the pH values and even temperature also act as growth limiting factors. This method is employed more often than turbidostat method because of fewer mechanical problems and presence of less amount of unused medium in the harvested culture (Fig. 2.14).
However, continuous fermentations have certain advantages and limitations which are as follows:
(a) Merits:
1. The fermenter is continuously used with little or no shutdown time.
2. Only little quantity of initial inoculum is needed and there is no need of additional inoculum.
3. It facilitates maximum and continuous production of the desired product.
4. There is optimum utilization of even slow utilizable substances like hydrocarbons.
(b) Demerits:
1. Possibility of contamination and mutation because of prolonged incubation and continuous fermentation, are more.
2. Possibility of wastage of nutrient medium because of continuous withdrawal for product isolation.
3. The process becomes more complex and difficult to accomplish when the desired products are antibiotics rather than a microbial cells.
4. Lack of knowledge of dynamic aspects of growth and synthesis of product by microorganism used in fermentation.
(c) Applications:
Continuous culture fermentation has been used for the production of single cell protein, antibiotics, organic solvents, starter cultures etc. (table 2.2).
Pilot plants or production plants have been installed for production of beer, fodder yeast, vinegar, baker’s yeast. A wide variety of microorganisms are used for this type of fermentation (table 2.3).
Type # 3. Fed Batch Fermentation:
It is a modification to the batch fermentation. In this process substrate is added periodically in instalments as the fermentation progresses, due to which the substratum is always at an optimal concentration. This is essential as some secondary metabolites are subjected to catabolite repression by high concentration of either glucose, or other carbohydrate or nitrogen compounds present in the medium.
For this reason, the critical elements of the nutrient medium are added in low amount in the beginning of the fermentation and these substrates continue to be added in small doses during the production phase. This method is generally employed for the production of substances such as penicillin. Yoshida (1973) introduced this term for the first time for feeding the substrates to the medium as the nutrients are exhausted, so as to maintain the nutrients at an optimum level.
The fed-batch fermentation may be of three types:
(i) Variable Volume Fed Batch Culture:
The same medium is added resulting in an increase in volume.
(ii) Fixed Volume Fed Batch Culture:
A very concentrated solution of the limiting substrate is added at a very little amount resulting in an insignificant increase in the volume of medium.
(iii) Cyclic Fed Batch Culture:
As it is not possible to measure the substrate concentration by following direct methods during fermentation, which is necessary for controlling the feeding process, generally indirect methods are employed. For example – in the production of organic acids, the pH value may be used to determine the rate of glucose utilization.
1. Production of high cell densities due to extension of working time (particularly growth associated products).
2. Controlled conditions in the provision of substrates during fermentation, particularly regarding the concentration of specific substrates for e.g. the carbon source.
3. Control over the production of, by products or catabolite repression, effects due to limited provision of substrates solely required for product formation.
4. The mode of operation can overcome and control deviations in the organism’s growth pattern as found in batch fermentation.
5. Allows the replacement of water loss, by evaporation.
6. Alternative mode of operation for fermentations dealing with toxic substances or low solubility compounds.
7. Increase of antibiotic marked plasmid stability by producing the correspondent antibiotic during the time span of the fermentation.
8. No additional special piece of equipment is required as compared with the batch fermentation.
9. It is an effective method for the production of certain chemicals, which are produced at optimum level when the medium is exhausted like penicillin.
1. It is not possible to measure the concentration of feeding substrate by following direct methods like chromatography.
2. It requires precious analysis of the microorganism. Its requirements and the understanding of its physiology with productivity is essential.
3. It requires a substantial amount of operator skill for the set-up of fermentation and development of the process.
4. In a cyclic fed batch culture, care should be taken in the design of the process to ensure that toxins do not accumulate to inhibitory levels and that nutrients other than those incorporated into the fed medium become limited also, if many cycles are run. The accumulation of non-producing or low producing variants may result.
5. The quantities of components to control must be above the detection limits of the available measuring equipment.
Fed-batch with recycle of cells can also be used for specific purpose such as ethanol fermentation and waste water treatment.
At present following products are being produced under fed batch culture:
1. Production of baker’s yeast.
2. Penicillin production.
3. Production of Thiostrepton by Streptomyces laurentii
4. Production of industrial enzymes, histidine, glutathione (Brevibacterium flavum), Lysine (Corynebacterium glutamicum)
(c) Applications:
1. It facilitates in avoidance of repressive effect.
2. It has control over organisms growth rate and O2 requirement.
3. In maintaining concentration of both the biomass and non-limiting nutrient substrates constant.
4. Production phase may be extended under controlled conditions and overcome problems associated with the use of repressive rapidly metabolized substrates.
5. Shift in growth rate may provide an opportunity to optimum product synthesis.
6. It facilitates to overcome viscosity problems or its toxicity at higher concentration.
Type # 4. Anaerobic Fermentation:
A fermentation process carried out in the absence of oxygen is called as anaerobic fermentation. There are two types of anaerobic microorganisms viz, obligate anaerobic microorganisms and facultative anaerobic microorganisms. The former like Clostridium sp. cannot withstand oxygen or remain active only in the absence of oxygen.
They remain active in the absence of oxygen and produce optimum amount of the desired product. The facultative anaerobes like lactic acid bacteria are able to withstand small amount of oxygen. However, certain organisms like yeast require an initial aeration to build up high cell yield before anaerobic conditions are created.
Anaerobic conditions in the fermenter are created either by withdrawing the oxygen present in the head space by an exhaust pump and pumping some inert gases like nitrogen, argon etc. or by flushing it out, by the emergence of certain gases like carbon dioxide or hydrogen (Fig. 2.4).
Stationary medium and viscous medium also creates anaerobic conditions. Sometimes in order to create anaerobic condition, medium is inoculated at the bottom of the fermenter soon after sterilization.
(a) Merits:
1. Production of economically valuables byproducts like carbon dioxide and hydrogen gas during anaerobic fermentation, which may fetch some profits to the manufacturers.
(b) Demerits:
1. Manufacturers may have to spend more money in providing extra provisions to the fermenter like exhaust pump in order to enforce anaerobic conditions.
2. It requires special media like viscous media whose preparation requires certain costly chemicals.
Type # 5. Aerobic Fermentation:
A fermentation process carried out in the presence of oxygen is called as aerobic fermentation. In most of the commercial processes and majority of the products of human utility are produced by this type of fermentation.
Fermentation can be surface culture or static and submerged.
Type # 6. Surface Fermentations:
Surface fermentations are those where the substratum may be solid or liquid. The organism grows on the substratum and draws the nutrients from the substratum. These types of fermentations are desirable where the products are based on sporulation. But it has several disadvantages such as it exposes the organism to unequal conditions, both oxygen and nutrients.
Type # 7. Submerged Fermentations:
Submerged Fermentations are those in which the nutrient substratum is liquid and the organism grows inside the substratum. The culture conditions are made uniform with the help of spargers and impeller blades. Most of the industrial fermentations are of this type. The substratum which is in a liquid state and such medium is also called as broth.
Type # 8. Solid Substrate/State Fermentation:
Solid state (substratum) fermentation (SSF) is generally defined as the growth of the microorganism on moist solid materials in the absence or near the absence of free water. In recent years SSF has shown much promise in the development of several bioprocesses and products, SSF has been ambiguously used as solid-state fermentation or solid-substrate fermentation.
However, it is proper to distinguish between two processes. Solid substrate fermentation should be used to define only those processes in which the substrate itself acts as carbon source occurring in absence or near absence of free water. On the other hand, the solid state fermentation is that fermentation which employs a natural substrate as above or an inert substrate used as solid support. Solid substrate fermentation are normally many step process involving.
SSF has a long history and some of the main events are précised in table 2.4.
Comparison of solid state and submerged fermentation is given in table 2.5.
Based on the need for aeration and agitation, SSF can be divided into two groups:
(a) Fermentation without agitation.
(b) Fermentation with occasional or continuous agitation.
Second group can be further divided into:
(i) Fermentation with occasional agitation, without forced aeration.
(ii) Fermentation with slow continuous agitation with forced agitation.
(iii) Pretreatment of a substratum that often requires either mechanical, chemical or biological processing.
(iv) Hydrolysis of polymeric substrates such as polysaccharides and proteins.
(v) Utilization of hydrolysis products.
(vi) Separation and purification of end products.
(vii) Fermentation with occasional agitation and forced aeration.
(viii) Fermentation with slow continuous agitation and forced aeration.
Several types of fermenters have been used for solid state fermentation. Laboratory studies have generally been carried out in flasks, beakers, Roux bottles, petri dishes, glass jars and columns. Inoculum is added after substrate autoclaving and incubated without any agitation and aeration.
For large-scale SSF bioprocess, three types of fermenters are in operation:
(a) Drum Fermenter:
It basically consists of drum type vessel usually equipped with a rotating device and arrangements for air circulation (Fig. 2.15a). The air inlet pipe may run parallel to the bottom or center or it may branch at several points over the whole length of the drum to facilitate air distribution which is normally attained by forced aeration, thus achieving the mixing of the fermenting substratum. Growth of the microorganism in this type of fermenter is considered to be better and more uniform than the tray fermenter.
(b) Tray Fermenter:
Tray fermenters are the simplest and can be constructed using wood, metals or plastic material. The bottom of tray is perforated in such a way that it holds substrate and allows aeration (Fig. 2.15b). Kofi fermentation has traditionally been carried out in tray fermenter. Tray fermenter, however, require a large operational area and labour intensive. Their design does not lead readily to mechanical handling. The substrate requires separate sterilization.
(c) Column Fermenter:
Column fermenter consists of a glass or plastic column with lids at both ends. It may be fitted with a jacket for the circulation of water to control the temperature of fermenting substrate. Alternatively, the whole column may be placed in temperature controlled water bath. Usually air is circulated from bottom to top (Fig. 2.15c).
The column may be vertical or horizontal as per convenience. Bed reactor is simple in design in which humidified air is pumped into substratum and the used waste gases goes out through the outlet provided continuous agitation with forced air to prevent adhesion and aggregation of substrate particles. These systems are very useful for biomass production for animal feed.
Microorganisms associated with solid substrate fermentation are those that tolerate relatively low water activity down to 0.7. They may be employed in the form of monocultures as in mushroom production e.g. Agaricus bisporus. Dual cultures e.g. straw conversion using Chaetomium cellulolyticum and Candida tropicalis. Mixed cultures as used in compositing and the preparation of silage where the microorganisms may be indigenous or added as mixed starter cultures.
For some fermentation, SSF is desirable because of following reasons:
1. In several productions, the product formation has been found superior in solid culture process.
2. The most commonly used microorganisms in the production of secondary metabolites are fungi and actinomycetes and the mycelial morphology of such organisms is ideal for their invasive growth on solid and insoluble substrates.
3. The fungal morphology is responsible for considerable difficulties in large scale submerged processes. These include highly viscous non-Newtonian broths and foam production. This results in very high power requirements for mixing and oxygen transfer. The presence of chemical antifoam in fermentation broth reduces oxygen transfer efficiency and can lead to problems in the product recovery.
4. In some processes the final product is required in solid form, such as antibiotics in animal feed.
5. The capital cost of overall production process is claimed to be significantly less.
6. The yields of certain secondary metabolites such as aflatoxin B1 and ochratoxin A obtained from liquid culture were found to be very poor. This led to the use of SSF to get higher yield of mycotoxins (100 g).
7. The fungus possess tremendous turgor pressure at the mycelial tips.
8. Microbial cells attach to solid substrate particles and completely surrounds the particle in mycelial webs.
9. It provides optimum quantity of water (aw) for growth.
10. Crude substrates can be used as the organisms can tolerate high concentration of metal ions and mineral ions.
11. Overcome catabolite repression and can be provided high substrate concentration.
12. Enzymes become extracellular otherwise intracellular in SMF. E.g.- Galactase, tannase and invertase.
13. Metabolite production phase is long.
14. Co-production of carbohydrates and proteases.
15. Enzymes produced by this will be with better properties and extra desirable components.
16. Fermentation of straw eliminates costly centrifugation and dewatering.
17. Lower capital and recurring expenditure.
18. Low waste water output/less water need.
19. Reduced energy requirement.
20. Absence of foam formation.
21. Simplicity.
22. High reproducibility.
23. Simpler fermentation media.
24. Lesser fermentation space.
25. Absence of rigorous control of fermentation parameters.
26. Easier aeration.
27. Economical to use even in smaller scales.
28. Easier control of contamination.
29. Applicability of using fermented solids directly.
30. Storage of dried fermented matter.
31. Lower cost of downstream processing
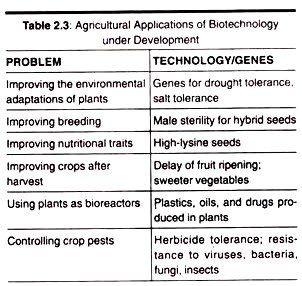
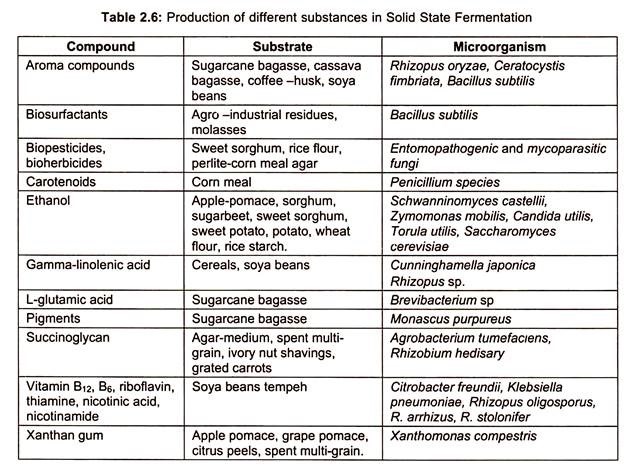
Some of the substances produced by SSF are precised in table 2.6: