Downstream Process in Fermentation [with methods such as precipitation methods].
The recovery and purification of fermentation products is one of the most important aspects of industrial fermentation processes. The selection of suitable process of recovery and purification depends upon the nature of the end product, their concentration, the by-products present, the stability of the product and degree of purification.
However, the selected procedure must be quick, simple, reliable, accurate and must measure the desired compound accurately from among the different chemicals that may be present in the growth medium. It is generally considered that the fermentation and product recovery are integral parts of an overall process. Because of interaction between the two, neither of them be developed independently, is necessary as this must not result in problems and unnecessary expenditure.
General Pattern of a Recovery Process:
The following flow chart shows the various stages that are generally involved in the recovery process of a biological product. The recovery of an extracellular product of a fermentation broth is taken as an example to describe the various stages involved in a recovery process (Fig. 2.23).
The first stage for the recovery of an extracellular product is the removal of the large solid particles and microbial cells by centrifugation or filtration process. The broth is then fractionated or extracted into major fractions. Fractionation is carried out by employing either ultrafiltration or reverse osmosis or adsorption/ion exchange/gel filtration or affinity chromatography or liquid – liquid extraction or two-phase aqueous extraction based on chemical and physical properties of substances or precipitation.
The product-containing fraction is then purified by fractional precipitation. Further, more precise chromatographic techniques or crystallization process is employed to obtain a product, which is highly concentrated and devoid of any impurities (table 2.15).
Various recovery processes can broadly be classified into the following categories:
1. Physico-chemical processes.
2. Chromatographic partition processes.
3. Biological assays.
1. Physico-Chemical Processes:
Though there are many physico-chemical recovery processes employed for the isolation of fermentation products, major of them are described here. However, it is to be remembered that the actual choice of recovery process largely depends upon chemical reaction or chemical analysis involved because the fermentation broth contains many compounds in addition to the desired one.
(a) Turbidity Analysis:
This technique is employed for the determination of cell yield or biomass, which is the primary product of the fermentation process. Centrifugation and determination of packed cell volume is the simplest method by which estimation of cell yields of a microorganism can be made quickly. Fermentation broth with the cells is centrifuged in graduated centrifuge tubes and the volume of sediment cells are measured in centimeters.
The other method employed for measuring the cell yields is the turbidity analysis of a fermentation broth, containing little insoluble debris other than cells. The cells present in the growth medium are diluted to a turbidity range and the cell suspension is exposed to a range of visible light or a monochromatic light with wave-length, (660 nm) in a colorimeter. Quantitative cell measurements are made as optical density by deflection of light that is caused by the microbial cells present in the diluted suspension.
For determining number of cells, trubidimetric measurements of cell numbers are usually standardized against some other procedures, such as plate count. A standard curve is then drawn with the help of values of optical density of plate count which may be derived from a series of dilutions of the cell suspension. Once the standard curve is prepared, it is easy to determine the cell number by converting reading of optical density.
(b) Titration and Gravimetric Analysis:
This technique is generally employed for the recovery of organic acids and the choice of recovery method varies according to the nature of the acid. For example the amount of an organic acid like lactic acid produced in fermentation can easily be determined by adding bromothymol blue or other pH indicating dye to a sample fermentation broth, followed by titration with an alkali of known concentration.
If the acid formed in a fermentation broth is able to form an insoluble salt with an alkali it can be isolated by precipitation, which can be washed, dried and weighed. Volatile acids with low molecular weight can be isolated by direct distillation of fermentation broth and distillate is analyzed by titration.
Similarly acids of high molecular weight can be separated from fermentation broth by adsorption to a suitable anion exchange resin and subsequent elution.
(c) Spectrophotometry Determinations:
Different types of spectrophotometers are used during spectrophotometric determinations. The difference in these analytical instruments lies in the kind of light absorbed. Visible light spectrophotometers absorb visible light in the range of 350 to 1000 nm. They measure the amount of light at a specific wavelength which is absorbed as light beam passes through a colored solution.
Ultraviolet spectrophotometers absorb light within the wavelength range of 200 to 380 nm, while in a fluorescent spectrophotometer the amount of light emitted by a fluorescent compound is measured.
Fermentation products which are colored can be measured directly by visible light spectrophotometers. A specific wavelength of visible light is allowed to be absorbed by a fermentation product and the absorption of light is measured as optical density. A standard curve is prepared with the help of the optical density of known concentration of a pure concerned chemical. By substituting the values of optical density of the fermentation product in the standard curve, the amount of fermentation product can be determined.
The colorless fermentation products are made coloured by treating with a colouring reagent. For example – amino acids are generally colorless chemicals but they develop purple colour when treated with ninhydrin under suitable conditions. The amount of fermentative production of amino acid is then determined by following the method described above.
Some fermentation products are basically colourless and do not develop colours even after chemical treatment. Determinations of such products are made with the help of ultraviolet spectrophotometers. By allowing them to absorb wavelength of ultraviolet light and measuring the optical density of the absorbed light.
Still some fermentation products like riboflavin fluoresce when exposed to ultraviolet light. The intensity of fluorescent light is measured by employing fluorescent spectrophotometers or fluorometer. By determining the optical density of the fluorescent light, the amount of fermentation product can be determined. The principles of analysis for ultraviolet and florescent spectrophotometers are similar to those for visible spectrophotometers.
2. Chromatographic Partition Determinations:
The term chromatography was originally used in earlier times for a technique to separate coloured compounds. Although partition is the predominant cause of separation, other factors are also involved though in a minor fashion.
Employment of partition chromatography on paper or thin layer plates, not only made marked strides in the fermentation research and technology, but also allowed to detect and identify many types of new fermentation products even though they are in extremely smaller amounts. An unknown compound can easily be detected with the help of this technique by comparing the partition value with already known compounds.
The basic principle involved in the partition chromatography is the continuous partition of the solute or the sample between a stationary phase, such as paper or the silica gel thin layer plates, and a mobile phase consisting of a mixture of solvents, as these solvents move across the paper or silica gel layer.
Basically there are eight types of partition chromatography:
(i) Paper chromatography,
(ii) Thin layer chromatography,
(iii) Column Chromatography
(iv) Ion Exchange Chromatography
(v) Adsorption Chromatography
(vi) Affinity Chromatography
(vii) Gel Filtration
(viii) Gas Chromatography
(i) Paper Chromatography:
If a cellulose paper is used as a stationary phase it is called as paper chromatography. Water soluble compounds are generally separated by employing paper chromatography.
In this technique the fermentation product to be separated is applied as series of spots about ¾ apart along a line, about few centimeters away from one end of a rectangular filter paper. Sometimes sample is also applied as a single spot on the paper. The paper is kept dipped in a mixture of solvent system consisting of a hydrophobic and a hydrophilic solvent. The solvent mixture starts ascending the paper and thereby wetting it by capillary action.
But the cellulose, which is the component of a paper, can absorb only the hydrophilic solvent and not the other. Thus there exists a phase separation at the micro-level. The compounds present in the fermentation broth get gradually separated when the solvent front reaches the spot, which contains the fermentation products to be separated. As the solvent front moves further the fermentation products get separated as consolidated spots on the filter paper.
For carrying out the solvent migration on the filter paper, (also called as chromatogram,) it is placed in a closed tank so that its atmosphere becomes saturated with the components of solvent system and helps in the perfect separation of the product. The developed chromatogram is removed from the tank and allowed air dry to remove extra solvent. The separation is measured in terms of a unit called Rf (Resolution factor) which is described as relative to front.
Its value is calculated by the following formula:
The Rf value of a given compound in a particular solvent system is constant under given set of physical conditions such as temperature, pH etc. and this can be used to identify the unknown compound.
Based on the method adopted, the processes of paper chromatography are classified into following categories:
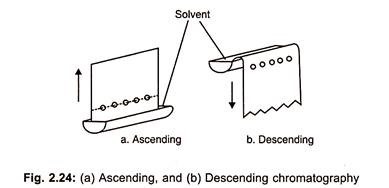
(a) Ascending Paper Chromatography:
In this method, the solvent system is placed as a shallow layer at the bottom of the tank and the filter paper strip is suspended in the solvent system in such a manner that the spotted sample does not dip in the solvent system, so that solvent ascends the paper (Fig. 2.24 a).
(b) Descending Paper Chromatography:
In this method, a trough containing solvent mixture is placed near the top of the tank and spotted chromatographic paper is placed in this trough so that the solvent migrates down the paper (Fig. 2.24 b).
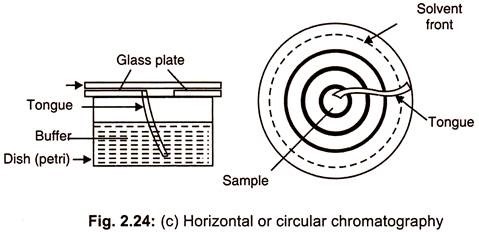
(c) Horizontal or Circular Chromatography:
In this method, a circular filter paper is chosen and a hole is made at its center. A wick placed in the central hole elevates the solvent to the chromatograph from a reservoir present beneath the sheet (Fig. 2.24 c) so that solvent migrates all round from the centre. This is also called as disc chromatography.
(d) Two-Dimensional Chromatography:
It is a modified or improved method of chromatography. It is employed in such situations where there are many compounds present in the sample and the individual spots on the chromatograph tend to coalesce (Fig. 2.25).
A single spot of the sample is applied at one corner of the rectangular filter paper and solvent migration is allowed across the paper. This moves out individual compounds along a line from the origin in the direction of solvent migration. Solvents are then evaporated by drying filter paper, it turned 90 degrees and placed in a second different solvent system.
Solvent migration with the second solvent system moves individual compound across the filter paper at an angle of 90° from the original direction of travel. This two dimensional chromatography allows the use of two different, even contrasting solvent system on a single chromatographic paper and separates the compounds more clearly.
However, it has certain major disadvantages are:
1. Only a single spot can be applied on the filter paper.
2. It is difficult to interpret the Rf values, as the values are associated with two different directions of solvent migration. But, it is still useful in the separation of compounds with small differences in their Rf values.
(ii) Thin Layer Chromatography:
Thin Layer Chromatography (TLC) is similar in most respects to paper chromatography except the usage of a thin glass plate in place of filter paper. A thin glass plate is coated with a thin layer of silica gel or aluminum oxide, which acts as a stationary phase. Calcium sulfate or starch is generally incorporated into the stationary phase compound, which helps in binding the latter to the glass plate surface.
The layer is allowed to dry at this stage, it is called as chromatogram. Fermentation broth sample is applied as a series of small spots approximately ¾ inch apart along a line about one inch from one end of the glass plate. The spots are allowed to dry at room temperature or heated with a stream of warm air or an infrared lamp.
After the spots have dried up glass plate is kept in a tank, saturated with solvent mixture, in such a way that spot do not dip in solvent mixture. The solvent mixture is then allowed to migrate through the chromatogram. The individual compounds present in fermentation broth get separated based on their molecular weight as consolidated spots on the chromatogram, when solvent mixture migrates through it. For precise analytical determinations the temperature at which solvent migration occurs must be carefully controlled. When solvent migration is done TLC plates are allowed to air dry.
The compounds thus separated on the chromatogram can be detected with the help of suitable spray reagent or made to absorb ultraviolet light. Amino acids can be detected by spraying the chromatogram with ninhydrin to produce purple coloured spots. Radioactive compounds can be located by exposing the chromatogram to x-rays.
The separated compounds on the chromatogram can be identified by calculating the Rf values and subsequently comparing the values with the known compounds. Biologically active compounds such as antibiotics can be detected by placing the chromatogram for short period on the surface of an inoculated agar plate.
(iii) Column Chromatography:
In this method, a vertical tube made up of glass or polyacrylate plastic is provided with an inlet and an outlet. The inlet is connected to reservoir containing a solvent to have a continuous flow of the solvent, while the outlet is fitted with rubber tubing to collect the eluting compounds. The column is fitted in the upright position and its bottom is sealed with a glass wool, which supports the stationary phase, which is gradually packed up to ¾ of the column.
The stationary phase is made of either gel or adsorbent or resin. The stationary phase is prepared in the form of a thick suspension and is called as slurry. After the slurry settles well in the column, the fermentation broth is applied at its top end to a height of 5-10 cm. The solvent mixture, which acts as a mobile phase, is made to flow through the column from its reservoir (Fig. 2.26).
As the solvent mixture moves through the column the products present in the fermentation broth get separated as the products emerge from the column outlet they are analyzed, identified and calculations with regard to their quantity is also made by employing one of the several appropriate analytical methods available like fluorescence detectors, voltameters, refractometric detectors, conductivity detectors etc. This technique is used for the separation of proteins and related compounds.
(iv) Ion Exchange Chromatography:
Macromolecules with ionic groups can be separated by ion exchange chromatography. The macromolecules are made to adsorb a carrier and are eluted by a solvent of defined strength. The separation mainly depends upon the nature of the macromolecule and the ionic strength of the eluting solvent.
This technique is generally used for the purification of antibiotics from fermentation broth, purification of proteins. The ionic material like Dowex, HCR and OCR, Amberlite, IR and IRC are widely used for cation exchange. Anion exchangers used are Dowex, SAR and MSA, Amberlite, IRA and Lewatit M.
(v) Adsorption Chromatography:
In adsorption chromatography separation of the contents of biological material is achieved due to hydrophilic and hydrophobic reaction between them. Elution and fractionations are accomplished by means of solutions of higher or lower polarity or strength. Silicates, alumina, activated carbon, cross-linked dextrans and hydroxylapatite are generally employed as adsorption material.
(vi) Affinity Chromatography:
It is a specific method for purification of biological material. The desired biological material binds specifically and reversibly to a ligand, which has been fixed to an inert carrier. For example – the Nucleotide Adenine Dinucleotide (NAD) can be purified by allowing it to bind to a carrier containing a dehydrogenase enzyme. The antibiotic bacitracin can be used as a ligand and to isolate aspartine, serine, cystine and metalloproteases from a crude mixture.
(vii) Gel Filtration:
In this technique, a gel is used as filtering agent and molecules of different size can be separated by allowing them to pass through the gel with different pore size. As the large molecules cannot penetrate the gel and pass directly through the column, finally elute out along with the mobile front, whereas small molecules penetrate deep into the gel and hence their mobility is inhibited from the solvent front.
Standard curves can be developed which relate molecular weight to the elution position. This technique is mainly used at the industrial scale to eliminate salts and separate low molecular weight impurities. It is also used to purify proteins like insulin or interferon, at a smaller scale industrial production. The Biogel types P and A from BioRad and the Sephadex series S, G and LH from Pharmacia are the gels generally employed in gel filtration technique.
(viii) Gas Chromatography:
In this technique fermentation products are first volatilized from the fermentation broth and then separated. The product to be separated from the fermentation broth is injected into the gas chromatograph and is converted into its gaseous state by heating.
The gas thus formed is pushed by a stream of inert gas through a partition column, and compounds get separated while passing through the column and are detected as they come out of the column by employing proper detecting system. Quantitative assay is obtained by comparison of the areas under peaks on plots of the data with corresponding areas for various quantities of reference compounds.
Determining the effect of a fermentation product or an enzyme on the growth and metabolic activities of test organisms is called biological assay or bioassay. Therefore, it is an indirect method of assessing the quality of a fermentation product. Biological assays are, therefore, difficult to perform, less reproducible and usually do not give accurate results.
The microorganisms employed in these assays are called as test organisms. Strains of microorganisms occurring in nature or artificially mutated ones may be employed as test organism. Various categories of microorganisms are used as test organisms. For example, bacteria for assaying amino acids, antibiotics and vitamins; fungi for assaying vitamins and trace elements; yeasts for assaying vitamins and antibiotics.
A microorganism that is employed in a biological assay should possess the following characters:
1. It should genetically be stable and should not exhibit any genetic change in response to the test chemical.
2. It should respond in a graded manner to the test compound and not to other compounds.
3. It should not be a pathogen and should grow on simple medium.
4. It should preferably be aerobic or facultative aerobic but not an anaerobic.
5. It should grow well at a pH that does not affect the stability or toxicity of the fermentation product under assay.
Biological assays can be categorized into four general groups.
They are:
(i) Diffusion assays,
(ii) Turbidimetric assays,
(iii) Metabolic response assays and
(iv) Enzymatic assays.
(i) Diffusion Assay:
In this assay, the fermentation product to be assayed is allowed to diffuse through medium in a radial fashion from a pad or cup and the adjacent growth of the test organism is either retarded or stimulated. The diameter of this area reflects the concentration of the compound being assayed and the amount of the product is determined by measuring and comparing the area with the various known concentrations of reference compounds.
There are two types of diffusion assays:
(a) The cylinder method and
(b) Paper disc method.
(a) Cylinder Method:
About 10 ml of molten agar medium is placed in a sterilized petri plate and is allowed to solidify. About 5 ml of the same or a different medium inoculated with a test microorganism is added above the solidified agar medium and is allowed to solidify. This layer is called seeded agar layer. Several small metal, glazed porcelain or glass cylinders are set on the agar surface.
The cylinders are filled with appropriate dilutions of the solution to be assayed and are incubated for a specific period of time at constant temperature. The diameter of the zone of stimulated or retarded growth is then measured in millimeters. The concentrations of the solution under assay are determined by comparison with a standard curve prepared from the inhibition or stimulation zone.
(b) Paper Disc Method:
Sterile filter paper discs of generally 12.8 mm diameter are used in this method. Each disc is treated with approximately 0.1 ml of reference compound. These treated discs are then placed on the surface of seeded agar medium and incubated as described for the cylinder assay. The calculations of assay results are similar to those for the cylinder assay.
(ii) Turbidimetric Assays:
In this method, the effect of the fermentation product under test in a liquid culture is measured as an increased or decreased turbidity associated with the growth rate or total growth of the microorganism.
A suitable sterilized medium is taken in series of sterilized test tubes and graded amounts of the product to be assayed are added. The tubes are inoculated with an equal amount of vigorously growing young culture of the test organism and then incubated for a predetermined period of time at a constant temperature.
The length of incubation period depends on the type of growth measurements, that is, whether growth rate or total growth of test microorganism. For measuring the growth rate, the turbidity of the culture at some point during logarithmic growth is determined, while for measuring the total growth of organism the maximum stationary growth phase is determined.
The relative turbidity produced in the test tubes can be determined either visually or by employing a spectrophotometer. Readings are made as optical densities or absorbance. The optical density is plotted against the concentration of the standard to obtain a standard curve. The concentration of the unknown product is determined by comparing its optical density with the standard curve. (Fig. 2.29 a and b).
(iii) Metabolic Response Assays:
These assays are similar to turbidimetric assays except that, instead of measuring the effect of the fermentation product on the rate of growth or the total growth of the test organism, the effect on some metabolic reaction that test organisms carries out during growth are measured. A few metabolic reactions used for assay of this type are acid production, carbon dioxide evolution, oxygen absorption and enzyme dehydrogenase activity.
(iv) Enzymatic Assays:
Enzymatic assays are more specific. They are able to detect quantitatively in extremely minute amounts of fermentable products and differentiate between biologically active and inactive forms of a compound. The required enzymes of these assays are derived from a commercial source or from a microbial culture or from other enzyme source.
The enzyme preparation is incubated with a sample of culture broth so as to cause some enzyme mediated changes in the fermentation product such as a partial decomposition with consequent formation of a measurable product.
For example – L-glutamic acid in a fermentation broth can be detected by adding cells of a pure culture of a strain of Escherichia coli, which contains the enzyme of glutamic acid decarboxylase. A little amount of toluene is also added to fermentation broth which facilities the release of enzyme from the cells of E. coli.
The test is carried out at an acidic pH 5.0. One molecule of CO2 is generally liberated from each molecule of glutamic acid. The CO2 liberated due to the degradation of L-glutamic acid by the enzyme glutamic acid decarboxylase collects in the atmosphere of the test tube containing the fermentation broth as a gas. Its concentration is calculated with a manometer such as Warburg respirometer.