In this article we will discuss about:- 1. Introduction to the Production Process of Ethyl Alcohol 2. Fermentative Production of Ethyl Alcohol 3. Applications.
Introduction to the Production Process of Ethyl Alcohol:
Ethyl alcohol has been produced on large scale for centuries. However, much study could not be accomplished because of hazards on human consumption. In 1865 Alcohol Act was passed thereby free sale of alcohol after its denaturing by adding methylated spirit was allowed. Early production was primarily used for human consumption. But today, apart from being used for human consumption, it is also used as universal solvent and as chemical raw material for the production of other industrial products.
Along with gasoline it is also used as motor fuel. Because of the above utilities the demand for alcohol increased enormously today, which lead to establishment of many distilleries throughout the world. Ethyl alcohol is produced, besides yeasts, by large number of bacteria and fungi (table 3.2).
The biochemical synthesis of alcohol by different microorganisms is depicted in Fig. 3.3.
Similarly, besides use of molasses, lignocelluloses can also be used as a sustainable substratum (table 3.3).
However, apart from fermentative production it is also produced by chemical processes primarily by catalytic hydration of ethylene.
Chemical conversion of lignocellulose by different fermentations are shown in table 3.5.
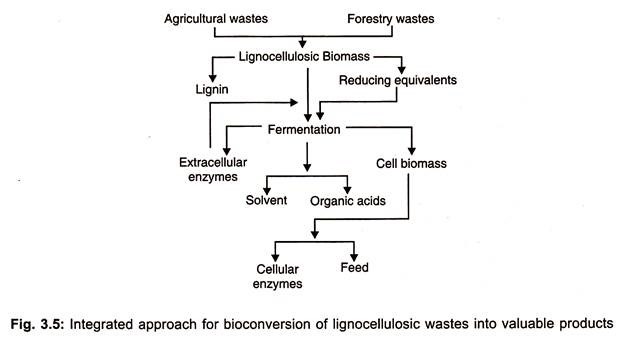
The whole process of bioconversion of lignocellulose into valuable sugars and other substances are illustrated in Fig. 3.5.
Fermentative Production of Ethyl Alcohol:
On commercial scale ethyl alcohol production process consists of four steps.
They are:
(i) Inoculum production,
(ii) Production medium,
(iii) Fermentation process, and
(iv) Harvest and recovery
(i) Production of Inoculum:
Both yeast and bacteria are used for the production of ethyl alcohol. Among the bacteria the most widely used organism is Zymomonas mobilis and among the yeasts Saccharomyces cerevisiae, Saccharomyces carlsbergensis, certain species of Candida and Mucor are also used, depending upon the raw materials available for ethanol production.
But high yielding and alcohol tolerant strains of S.cerevisiae are usually employed. Details of process for production of ethyl alcohol from different substrates are depicted in Fig. 3.3. High yielding strains of yeast are generally used for commercial production of ethanol. These are developed by genetic selection.
The strain of yeast that may be used for the industrial production should possess the following characters:
1. It should possess uniform and stable biochemical properties.
2. The ability to ferment a broad range of carbohydrate substrates rapidly.
3. It should yield large quantities of ethanol.
4. It should grow fast and should have high osmotolerance.
5. Low levels of by products such as acids and glycerol.
6. It should be tolerant to higher concentration of alcohol.
7. It should possess high temperature tolerance.
8. High cell viability for repeated recycling.
9. Appropriate flocculation and sedimentation characteristics to facilitate cell recycle.
However, the choice of microorganism employed in large scale ethanol production depends upon the type of raw material used. For example S. cerevisiae is employed when starch or maltose is used as raw material, when whey or sulphite waste liquor is used as raw material Candida utilis and C. albicans respectively is employed in the fermentation process. Production of ethanol from starch is shown in Fig. 3.3.
Inoculum Production Process:
A suitable pure strain of yeast is inoculated into a test tube containing approximately 10 ml of sterile medium. It is incubated at 28°C to 30°C till sufficient growth of yeast takes place. The medium employed for the preparation of inoculum and the fermentation process is generally similar.
1. After sufficient growth of the yeast occurs, the culture from the test tube is transferred to a flask containing approximately 200 ml of the medium. The flask is incubated at 28°C to 30°C until predetermined cell mass is formed.
2. The fully-grown culture from the flask is transferred to a glass container containing 4 liters of sterile medium and is incubated at 28° to 30°C till sufficient growth takes place.
3. The culture from the glass container is finally transferred to a small seed tank containing 40 gallons of sterile medium. Then seed tank is to be near the fermentation tank. The culture broth after incubation will be ready for inoculating into the production tank.
A large amount of yeast culture ranging from 8 to 10% volume of inoculum is required in the industrial production of ethanol. To achieve rapid growth of yeast and large amount of cell mass, high degree of aeration and agitation of the medium is required. The pH and temperature are adjusted at 4.8 and 28° to 30° C respectively during the growth of the yeast.
(ii) Preparation of Medium:
Composition of fermentation medium plays an important role in achieving optimum yield of ethanol. It should be prepared in such a way that it contains all the sources of materials that promote optimum growth of yeast and optimum yield of ethanol. Generally, the medium should possess carbon source, nitrogen source and growth factors.
(a) Carbon Source:
Different varieties of carbohydrates which are produced as waste products in agricultural industries can be used as carbon source.
They are grouped into the following categories depending upon their chemical nature:
1. Starchy material – potato starch, cereals like oats, wheat flour and corn starch.
2. Saccharide material – fruit juice, whey, molasses and hydrol.
3. Cellulose material – sulphite waste liquor, lignocellulose.
Molasses is generally used as the main carbon source in the preparation of fermentation medium. However, cane molasses is used in India because of its availability in large quantities from sugar industries. The raw material indicated above, require pretreatment in the form of saccharification. They are put to hydrolysis during saccharification due to which easily fermentable sugars like maltose and glucose are formed.
The optimum sugar concentration should be maintained between 10 to 18% in the fermentation medium. If beet molasses are used, biotin should be added to meet the biotin deficiency. If cane molasses is used as a carbon source, its sucrose concentration should be reduced to 10% by the addition of distilled water which is called a Wort. Higher sucrose concentration affects the growth of yeast adversely, while lower sucrose concentration makes the fermentation process uneconomical.
In recent times, lignocelluloses as source of carbon proved to be more economical and sustainable. Most of the plants after death are subjected to decomposition releasing fermentable sugars. Large amount of lignocellulose out of waste agricultural products can be converted to sugars by enzymatic hydrolysis. Annual production of these lignocelluloses around the world is given in table 3.3.
Composition of plant lignocellulosic materials on hydrolysis are depicted in table 3.4.
Integrated process of conversion of lignocellulose wastes into value added saccharides is presented in Fig. 3.5 and ethanol production from different substrates is depicted in Fig. 3.4.
(b) Nitrogen Source:
Variety of inorganic and organic nitrogenous compounds may be used as nitrogen source in the medium preparation. Ammonium sulphate is generally used as nitrogen source. Generally 0.15 g of ammonium sulphate is added to 2.5 gallons of molasses. The concentration of nitrogen should be carefully maintained as indicated above. Excess nitrogen promotes rapid growth of yeast cells and decreased production of ethanol.
(c) Growth Factors:
As most varieties of molasses, for example cane molasses, contain suitable concentration of growth promoting substances, hence there is no need for addition of any growth promoting substances separately in the preparation of the medium.
(d) pH:
The pH of the medium should be adjusted to 4.8 to 5.8 by adding sulphuric acid or lactic acid. As the medium becomes highly anaerobic at this range of pH, this inhibits the growth of contaminating bacteria. There is no need of sterilizing the medium. Further anaerobic fermentation of alcohol discourages growth of many microorganisms. However, pasteurization of the medium can be done.
(iii) Fermentation:
Ethyl alcohol can be produced by any one of the following processes:
1. Batch fermentation,
2. Continuous fermentation with cell, and
3. Continuous fermentation with cell recycling.
A comparison of the operating parameters of the above fermentation processes are indicated in table 3.5.
However, batch fermentation is more commonly employed for ethanol production. Production is carried out in a large fermenter with a volume of 600 cm3. About 30% of inoculum (cell density 3 × 106 ml-1) is generally employed. It is added to the fermenter by pumping or gravity. This addition of inoculum to the fermenter is called as pitching. The following environmental factors like incubation time and temperature are to be suitably maintained and controlled in order to achieve optimum yield.
The time required for the maximum yield of ethanol is 30 to 72 hours, which largely depends upon the specific gravity of the fermentation liquid. The fermentation is generally stopped at any hour when the specific gravity of the fermentation liquid becomes constant. At this stage, 6 to 8% of ethyl alcohol will be formed. Fermentation generally starts within few hours of yeasts inoculation. The process becomes rapid after 24 hours.
A temperature range of 25-30°C is favourable for ethanol production. However, as heat is evolved during the fermentation process, the temperature in the fermenter gradually increases and is controlled and maintained at the above indicated range by means of cooling coils or by spraying cold water around the fermenter.
Periodical agitation of the fermenter is also required for enforcing uniform cooling of the medium. If higher temperatures are not controlled, contamination of the fermentation broth may occur due to the growth of thermophilic bacteria and also result in the loss of ethanol due to surface evaporation.
When all the fermentation factors are optimum there may be production of 1.9 grams of ethanol one liter of medium per hour. About 90% of carbon source of the fermentation liquid is converted into alcohol.
(iv) Harvest and Recovery:
The cell mass is separated before distillation by either centrifugation or sedimentation. It is then distilled in analyzer and rectifier columns to get ethyl alcohol also called rectified spirit and fuel alcohol (higher alcohols). A mixture containing 95.6% ethyl alcohol and 4.4% water is obtained by fractional distillation.
After distillation, the spent wash and bottom sludge are drained out as distillery waste. The product is marketed as rectified spirit, denatured spirit or special spirit. Zymomonas mobilis produces upto 120 g ethanol per liter per hour.
The flow diagram for the production of ethanol is shown in the Fig. 3.6.
Some of the byproducts are depicted in Fig. 3.7.
Large distilleries employ, generally, special rectifier column called as coffey’s still which consists of two columns called as the analyzer and the rectifier.
(a) The Analyzer:
The analyzer is a vertical tower. It consists of columns arranged in a zigzag manner. The fermentation liquid is allowed to flow down the analyzer and simultaneously steam is allowed to move up the tower from its bottom. Alcohol present in the fermentation liquid vaporizes and its vapours collect at the bottom of the tower and are made to flow into the rectifier.
(b) The Rectifier:
Just like analyzer, the rectifier is also a vertical tower. It consists of specially designed fractionating columns with a number of chambers. The less volatile constituents (the slop and fuel oil) gradually condense and are drawn off from a higher point of the still. The temperature at the higher point of still is roughly equal to the boiling point.
The head products, which are very less, contain aldehydes, formic esters etc. owing to their greater velocity, pass out through the top of the column along with a small quantity of uncondensed alcohol. The higher alcohols are removed from the receiver for every two or three days.
Applications of Ethyl Alcohol:
1. It is extensively used in synthesis of variety of organic compounds and is a universal solvent.
2. As energy source in motor fuel cells.
3. Different byproducts are useful in a variety of ways.