In this article we will discuss about:- 1. Introduction to Acetic Acid 2. Biosynthesis of Acetic Acid 3. Fermentation Process.
Introduction to Acetic Acid:
Acetic acid (CH3COOH) is also called as vinegar. Vinegar fermentation is one of the oldest fermentations known to man. It is formed naturally due to spoilage of wine. Therefore, literally vinegar means “sour wine.” Technically vinegar is fermented food product consisting of about 4 g of acetic acid per 100 ml. Vinegar was produced only for local consumption until the middle ages.
The Romans and Greeks, who used diluted vinegar as refreshing drink, produced vinegar by leavening wine open to air. Industrially for the first time it was produced in open vats, which however, was a very slow process. But the process was increased many fold in the 19th century by surface fermentation technique in which trickling generators were used. This process is used still today.
From 1949, submerged process was employed in the fermentative production of vinegar. Presently both these processes that is surface fermentation and submerged fermentation are employed worldwide. The surface fermentation is used still today because of the better flavour of the product.
Biosynthesis of Acetic Acid:
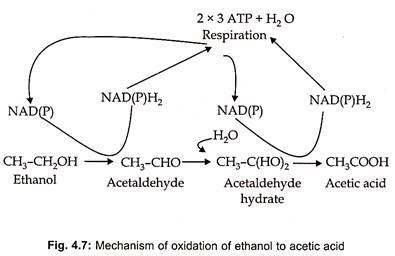
The production of vinegar actually involves two fermentation processes- the first utilizing yeast to produce alcohol from sugar and the second utilizing acetic acid bacteria to oxidize ethyl alcohol acetic acid through acetaldehyde (Fig. 4.7).
The microbial oxidation of ethanol to acetic acid is an aerobic fermentation that has high oxygen requirement. Acetobacter bacteria are employed for industrial production of vinegar. Acetobacter bacteria can be divided into two groups – Gluconobacter and Acetobacter. Gluconobacter oxidizes ethanol to acetic acid, while Acetobacter oxidizes ethanol first to acetic acid and then to CO2 and H2O.
Species of the Acetobacter used commercially are Acetobacter aceti and A. pasteurianum. Similarly, Gluconobacter oxydans and its subspecies are employed in the commercial production of vinegar. Mixed cultures, sometimes appear during production even though pure culture is used especially in surface process.
Two oxidation steps occur during the conversion of ethanol to acetic acid. In the first step ethanol is oxidized to acetaldehyde in the presence of NAD or NADP and in the second step acetaldehyde is changed to acetic acid and render the catalytic action of the enzyme alcohol dehydrogenase (Fig. 4.7). In this oxidation one liter of 12% acetic acid is produced from one liter of 12% alcohol that is one mole of acetic acid is formed from one mole of alcohol.
Fermentation Process of Acetic Acid:
Commercially acetic acid is produced by two methods, surface fermentation process and submerged fermentation process. If materials with low alcohol content are used such as wine, whey, malt or cider there is no need of addition of any component to constitute a complete nutrient solution. However, if potato or grain spirits or technical alcohol is used, nutrients must be added to obtain optimal growth and acetic acid production. Nutrient concentration that is used in submerged fermentation is generally five times greater than surface fermentation.
(a) Surface Fermentation Process:
Trickling generator is generally used in this process (Fig. 4.8a).
It is made up of wood and has a total volume upto 60 m3 and its inner surface is lined with birch wood shavings. The starting material, that is, ethanol is passed into the generator from top which trickles through the birch wood shavings containing bacteria into bottom basin where the partially converted solution is cooled and pumped back again to the top of the generator and passed again through it.
Thus, the process is repeated again and again until 88-90% of alcohol is changed to acetic acid. The starting material should contain both acetic acid and ethanol for optimal growth of Acetobacter. However, ethanol supply is critical because if it is less than 0.2% (v/v) in solution the death rate of bacteria increases, but its concentration should not increase above 5%. Presently higher yielding strains are employed in vinegar fermentation which are able to yield 13-14% of acetic acid.
(b) Submerged Fermentation Process:
The production rate of ethanol with submerged fermentation process is ten times higher per cubic meter than the surface fermentation process. Other advantages include lower capital investment for production, 20% plant area required for installation, conversion to other mashes in a short time and low manual cost because of fully automatic control.
Material with low alcohol concentration such as fruits, wines and special mashes were first used in the initial stages of submerged fermentation process, which generally do not require aeration. But presently high yielding materials are employed which are capable of yielding 13% acetic acid. However, the process with such high yielding material requires high aeration upto 50 m3 oxygen.
Fermenters constructed with stainless steel are employed and they are stirred from bottom. Aeration is provided with a suction rotor, with the incoming air coming down through a pipe from the top of the vessel (Fig. 4.9).
Heat exchanger is provided to control the temperature along with foam eliminators. Domestic vinegar is produced through semi continuous fully automatic process under continuous stirring and aeration. The starting material 100 ml of 5% ethanol are used in the process to get 7-10 g acetic acid/100 ml.
The fermentation process is carried upto 35 hours at 40°C temperature. The ethanol concentration is continuously measured and when its concentration goes down to 0.05%-0.3%. 50%-60% of the solution is removed and replaced with a new mash with 0.2 g acetic acid/100 ml and 10-15% ethanol. The yield of acetic acid is about 98% in fully continuous process.
(c) Recovery:
The vinegar produced in a submerged fermentation process is turbid due to presence of bacteria. It is clarified by filtration. Plate filters and filter aids are generally used. After filtration K4[Fe(CN)6] is used to decolorize the final product, if required.
The submerged fermentations also utilize two different fermenter designs known as Acetator and Cavitator (Fig. 4.10).
Both these fermenters provide the high aeration levels required for acetic acid production. These intact pump recycle their own air so that a compressor is not required. This aeration approach is advantageous for acetic acid fermentation. Since it lessens the loss of ethanol and aromatic substances present in the raw materials. Automatic temperature control is applied to dissipate the heat resulting from the ethanol oxidation. These two fermenters differ in their mechanical operations and in the fermentation programmes.
The acetator usually operates in semi batch manner, while cavitator in nutrient solution and air is sucked down to a hollow tube extending from the liquid surface so that agitation and cavitation fermentation extends to the bottom of tube resulting in mixing of air with nutrient solution.