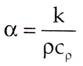In this article we will discuss about the rheological properties of food.
Properties of Solid Food:
The science of rheology deals not only with liquid food but with solid food as well. With solid food, such as fresh fruits and vegetables, scientists study the effects of deformation on the food products. Deformation is a permanent change in the shape of an object due to the addition of force. A force is an energy exerted on a substance that causes a change in shape or direction of the substance.
A force can change the shape of a solid food product or direct the flow of a liquid one. The relationship between the addition of force and the amount of deformation is linear in the beginning. That is, when the first units of force are applied to a solid food object, the resulting amount of deformation increases equally with each addition of force.
Eventually, with continued force, the object will reach the yield point, where deformation continues without the input of additional force. At this point, the change in shape of the object is permanent. Finally, the force will cause the food to split, crack, or break at a point known as rupture. Each substance will have a different rupture point.
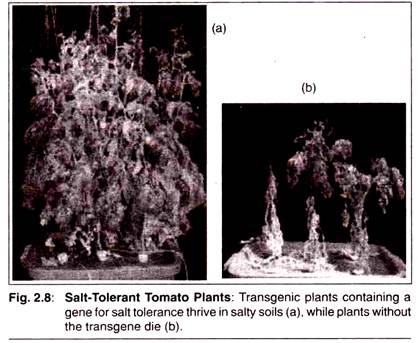
This will affect how the product is harvested, processed and shipped to the marketplace. With tomatoes, green tomatoes have the ability to withstand much greater forces during processing and shipping than red tomatoes, which rupture much more easily.
The evaluation of rheological properties of solid food can be divided into two broad classes:
1. Fundamental Test:
Fundamental test measure the properties that are inherent to the material and do not depend on the geometry of the test sample, conditions of losing, or the apparatus. Example of such properties includes elasticity, poisson ratio and relaxation time and shear modulus.
The fundamental tests as applied to solid food may again classified into two:
i. Those conducted under static conditions (quasistatic loading).
ii. Those conducted under dynamic condition.
2. Emperical or Imitative Tests:
These tests are used to determine properties such as puncture force and extrusion energy, where the mass of the sample, the geometry, speed of the test etc.
Elastic Behavior of Solid Food:
Hookean Solids:
Pure elastic behavior of solid is defined as a when a force is applied to a material, it will instantaneously and finitely deform and when the force is released the material will instantaneously returns to its original form. Such solid materials are called Hookean solids. Here the amount of deformation is proportional to the magnitude of the force. The rheological representation of this type of solid is a spring.
There are three types of moduli may be calculated for a Hookean solids depending upon the method of applying the force:
1. Modulus of elasticity (E)
2. Modulus of rigidity (G)
3. Modulus of bulkiness (K)
The modulus is calculated by the applying a force perpendicular to the area defined by the stress is called modulus of elasticity (E). The modulus is calculated by the applying a force parallel to the area defined by the stress is called modulus of rigidity (G). If the force is applied from all possible directions and the change in volume per calculation of original volume is termed as modulus of bulkiness (K).
Properties of Fluid Food:
The rheological properties of liquid food are based on flow and deformation responses when subjected to stress. Most fluid contains water and relative large amounts of dissolved molecular weight compounds (e.g. sugar) and no significant amount of polymers or insoluble solids.
The rheological properties of liquid food are altered based on the thermal properties such as density, heat capacity and thermal conductivity. The most important factors which determine the properties of liquid food are shear flow properties. These are based on shear rate and shear stress.
Shear stress denoted by the symbol o and is the stress component applied tangentially, it is equal to the force vector (a vector has both magnitude and direction) divided by the area of application. Shear rate denoted by the symbol y and is the velocity gradient established in a fluid as results of shear stress acting on it. Viscosity is the internal friction of fluid or its tendency to resist flow. It is denoted by the symbol |i for Newtonian fluids and q for non-Newtonian fluids.
The formula is given below:
Based on the rheological properties all liquids food can be classified in to two:
1. Newtonian fluids, and
1. Newtonian Fluids:
A Newtonian fluid (named after Isaac Newton) is a fluid whose stress versus strain rate curve is linear and passes through the origin. The constant of proportionality is known as the viscosity.
A simple equation to describe Newtonian fluid behavior is:
These fluids are containing only low molecular weight compounds (e.g. water and sugars) and that do not contain large concentrations of dissolved polymers (pectins, proteins and starches) or insoluble solids (insoluble fibers). Examples of Newtonian fluids include water, sugar, syrup, honey, edible oils, filtered fruit juices, pasteurized milk and carbonated beverages. A Newtonian fluid is represented graphically in Fig. 9.1. Graph A shows that the relationship between shear stress (T) and shear rate (y) is a straight line. Graph B shows that the fluid’s viscosity remains constant as the shear rate is varied.
2. Non-Newtonian Fluid:
A non-Newtonian fluid is a fluid whose flow properties are not described by a single constant value of viscosity, i.e. the shear rate-shear stress plot is not linear or the plot does not begin at the origin. The material exhibits time independent rheological behavior as a result of structural changes.
Many polymer solutions and molten polymers are non-Newtonian fluids, as are many commonly found substances such as ketchup, starch suspensions, paint, blood and shampoo. In a non-Newtonian fluid, the relation between the shear stress and the strain rate is nonlinear and it can be time-dependent.
Therefore a constant coefficient of viscosity cannot be defined. A ratio between shear stress and rate of strain (or shear-dependent viscosity) can be defined, this concept being more useful for fluids without time-dependent behavior.
In the case of certain fluid food are exhibited a particular property called as shear thinning. In such food the curve begins at the origin of shear stress-shear rate plot but is in concave upwards, i.e. an increasing shear rate gives a less proportional increase in shear stress.
These types of food are called as pseudoplastic food. Shear thinning is due to the breakdown of structural units in a food due to the hydrophobic forces generated during shear. Most non-newtonian food exhibits shear thinning behavior including mayonnaises, salad dressings, concentrated fruit juices and fruits and vegetables.
Properties of Granular Food and Powders:
The physical properties of granular materials and powders have direct influence on the transport of these types of food within a food processing operation.
The important properties of granular and powder food is discussed below:
1. Bulk Density:
For granular materials and powders, there are different types of density to be described.
The bulk density can be defined as follows:
The bulk density of any material is the overall mass of the material divided by the volume occupied by the material. Food powder will occupy variable volume of the space between the particles.
The bulk density measurement may be two types:
i. Loose Bulk Density:
It is obtained by careful placement of the granular materials in a defined volumetric space without vibrations, followed by the mass.
ii. Packed Bulk Density:
It would be measured by vibration of a defined mass until the volume is constant, then compute the bulk density.
The bulk density can be predicted by the following relationship:
2. Particle Density:
The density of the individual food particle is referred to as particle density and it is a function of gas phase (air) volume trapped within the particle structure.
The particle density is predicted by the following relationship:
Where the density of the gas phase or air (ρa) is obtained from standard table and the density of particle solid is based on the product composition. ea is the volume fraction of solids. Porosity (Ψ) is the ratio of the air volume to total volume occupied by the product powder.
The relationship of porosity to bulk density becomes:
Particle Size and Size Distribution:
An important property of a granular food or powder is particle size. This property has direct impact on the magnitude of the bulk density as well as the porosity. Particle size are measured by
using several different techniques, including sieve, a microscope, or a light scattering instrument.
The measurement of particle size of the granular material is given below:
Where d is the particle diameter, N is the number of particle with a given diameter and q and p are the parameters present in standard table.
The movement or flow of a granular food or powder is influenced by several properties of the material. A property of the granular food is directly related to flow is angle of response. This is a simple property to measure by allowing the powder to flow from a container over a horizontal surface. The height (h) of the mount of powder and the circumference of the mound (s) are measured.
So the angle response is calculated using the following equation:
The magnitude of this property will vary with density, particle moisture content and the particle size distribution of the granular powder.
Sedimentation Behavior:
Sedimentation behavior is another important property of fluid food items. Sedimentation is the tendency for particles in suspension or molecules in solution to settle out of the fluid in which they are entrained and come to rest against a wall. This is due to their motion through the fluid in response to the forces acting on them. These forces can be due to gravity, centrifugal acceleration or electromagnetism.
Sedimentation relies on gravitational force to effect separation of particles from a fluid because of a difference in density. The gravitational force is balanced by a drag force applied by the fluid. The concept of constant velocity, the thermal velocity and the sedimentation behavior is expressed by Stoke’s Law.
Vt = Thermal velocity, g = Gravitational acceleration, D = Particle diameter, ρp = Particle Density, ρf = Fluid density and μ = Fluid viscosity.
The equation can be used to measure viscosity when all other parameters are known. The sedimentation is mainly due to the thermal velocity is reduced from the Stoke’s value because of interaction among particles and increased fluid flow as the fluid is displaced by falling particles. Separation of particles from fluids can be intended to remove turbidity from the fluid. This process is called clarification. This process is common in treating water, wine and beer. In clarification, it is common to add chemicals that promote aggregation of particles and thus increase settling rates.
Observation of the height of the interface between settled solids and clear liquid at various times gives a settling curve. This curve is useful to determine rate of sedimentation. If the fluid foods are more concentrated with the addition of solid particles, this process is called thickening.
Thermal Properties of Frozen Food:
The proper knowledge of physical and thermal properties of food is essential for the designing of the food freezing equipment and processing. The determination of refrigeration requirements and freezing times can be calculated only when quantitative information on food properties are available.
During the freezing process, water changes gradually from liquid phase to solid ice. Since the properties of ice are different from those of liquid water the properties of food determined at temperature above freezing are not valid for sub-freezing conditions. The density, porosity and solute concentration have major effects on thermal properties.
The most dramatic changes in these properties are observed at temperature close to the freezing point. Thermal properties of food are important for predicting the energies and speed of freezing and thawing reactions. The thermal properties of frozen food are dependent on their water content and temperature.
Water and ice having large specific heat capacity and thermal conductivity in comparison with remaining components of food (protein, fat and carbohydrates). The properties of ice in the food increases as its temperature are lowered from the initial freezing point down to -30°C and lower.
The most important types of thermal properties of frozen food are:
(1) Density
(2) Thermal conductivity
(3) Enthalpy
(4) Specific heat, and
(5) Thermal diffusivity.
The density of a material is defined as its mass per unit volume.
Where, ρ is the density is the mass and V is the volume. The density of a food product is measured by weighing a known volume of the product. Since food products are different shapes and sizes, the accurate measurement of volume is a complicated process.
Thermal conductivity is the property of a material that indicates its ability to conduct heat. Ice is about four times thermally conductive than water. So the thermal conductivity of frozen food increases with decreasing temperature (as the ice content of the food increases). Lipids are poor conductors of heat and therefore, fatty food tends to have lower thermal conductivity than non-fatty food of the same water content.
Thermal conductivity of food mainly depends on the anisotrophy (non isotrophic) and porosity. Air present in the pores and cavity of food (e.g. bread) reduces thermal conductivity. Measurement of thermal conductivity has involved the use of both steady state and non-steady state methods.
(3) Enthalphy:
The enthalpy of frozen food is related to the energy (heat) that a freezer has to remove in order to lower the temperature of food from the unfrozen to the frozen state. This heat consists of two parts: the sensible and latent heat. The sensible heat can be sensed by measuring temperature because it is related to the energy stored in a substance by increasing the speed of its molecules.
The latent heat is related to the conversion of water in to ice. Water has high latent heat and sensible heat compared with other food components, (the specific heat capacity of water is about 4. 2 KJ/ kg/°C and of ice is 2. 1 KJ/kg/°C) so the amount of water in food has high influence on its enthalpy.
(4) Specific Heat:
Specific heat capacity or specific heat is the measure of the heat energy required to increase the temperature of a unit quantity of a substance by unit degree. It is commonly determined by the use of calorimetric methods.
Thermal diffusivity is the ratio of thermal conductivity to volumetric heat capacity.
The equation for thermal diffusivity is given below:
Where k is the thermal conductivity, ρ is density and Cρ is specific heat capacity Substances with high thermal diffusivity rapidly adjust their temperature to that of their surroundings, because they conduct heat quickly in comparison to their volumetric heat capacity.