Industrial production of amylases, glucose isomerase, pectinases, lipases, cellulases and proteases are described in this article.
1. Amylases:
Amylases are a complex group of enzymes that hydrolyse polysaccharides like starch and glycogen to glucose. During the hydrolysis of 1, 4-glycoside, linkages present in above polysaccharides are degraded which result first in the formation of short chain dextrins, then to maltose and glucose. Different enzymes involved in the degradation of starch to glucose are illustrated in Fig. 8.3 and precised in table 8.2a.
There are mainly two groups of amylases such as –
1. α -amylose and
2. β-amylose.
1. α -amylose – α -amylases are also called as 1,4 – α – glucan – glucano hydrolase. Extracellular enzymes hydrolyze 1,4 – glycosidic bonds. These enzymes are also called as endoenzymes as they split the substratum in the interior of the molecule in a random fashion. On the other hand,
2. β-amylose – β-amylases split the substratum from one end of the molecule in a successive fashion.
On every alternate 1, 4 glycosidic bond releasing maltose. These enzymes are also called exo amylases. The other enzymes involved in the degradation of starch include amyloglycosidase (Aspergilus niger and Rhizopus niveus), isoamylase and pullulanase (Klebsiella pneumoniae and Bacillus acidopullulyticus). The molecular weight of various α -amyloses do not differ considerably (table 8.3) and require calcium as a stabilizer.
α-amylases are secreted by many bacteria and fungi.
They are classified according to their:
1. Starch-liquefying or saccharogenic effect
2. pH optimum
3. Temperature range
4. Stability
Saccharogenic amylases produce free sugars (β-amylose and glucoamylase), whereas starch- liquifying amyloses (α-amylose) break down the starch polymer but do not produce free sugars.
Bacteria which produce α -amylase are Bacillus subtilis, B. cereus, B. amylo- liquefaciens, B. coagulans, B. polymyxa, B. stearothermophilus, B. caldolyticus, B. acidocaldarius, B. subtilis amylosaccharaticus, B. licheniformis, species of Lactobacillus, Micrococcus, Pseudomonas, Arthrobacter, Escherichia, Proteus, Thermomonospora and Serratia. Some α-amylase producing fungi are species of Aspergillus, Penicillium, Cephalosporium, Mucor, Candida, Neurospora and Rhizopus.
Although all the above bacteria and fungi are capable of producing α-amylase, the most important of them from which α -amylase are produced commercially through fermentation process include Bacillus amyloliquefaciens, B. licheniformis and Aspergillus oryzae. However, Bacillus are used much more extensively than those of Aspergillus. The most important areas of application for these two enzymes are precised in table 8.4.
Fungal α -Amylase:
Fungal α-amylase is produced commercially by employing either Aspergillus oryzae or Aspergillus niger. Stationary culture method is used when A. oryzae is employed, while submerged culture method is used when Aspergillus niger is employed.
The process of submerged culture method is only described here:
(a) Preparation of Inoculum:
Suitable and pure seed culture is generally selected as inoculum. In certain cases mutants capable of giving higher yield are selected as inoculum.
(b) Preparation of Medium:
The following medium is generally employed for submerged fermentation:
Amylase biosynthesis is inhibited when there is glucose in the medium. The medium is steam sterilized. The sterilized medium is passed into a production fermenter for α-amylase production.
(c) Fermentation Process:
A cylindrical fermenter made up of stainless steel is generally used in the fermentation process. It is equipped with an agitator, an aerating device, a cooling system and other ancillary equipment like a device for foam control, monitoring of pH, temperature and control of oxygen tension etc.
A sufficient quantity of pre-sterilized production medium is taken in the fermenter and is inoculated with spores of the selected species of the fungus. The spores are allowed to germinate and produce sufficient mycelium by controlling the fermentation conditions. Control of fermentation conditions plays a vital role in the success of the process, which include pH, temperature, aeration, agitation, oxygen supply etc.
The optimum pH for the fermentation is 7.0. Calcium carbonate is used as buffer to maintain pH. The fermentation process is generally operated at a temperature of 30 to 40°C. Aeration and agitation of the production medium is needed because of high viscosity of the medium due to the presence of mycelial mat.
(d) Harvest and Recovery:
The following steps are followed during the recovery of the enzyme after the completion of fermentation. In order to avoid denaturation of the enzyme, the fermentation broth is subjected to rapid cooling at 5°C temperature immediately and the enzyme is extracted.
1. Separation of fungal mycelium is accomplished by filtration of the refrigerated broth.
2. The suspended particles present in the broth are removed with flocculating agents like calcium phosphate.
3. The enzyme is precipitated, in order to get high degree of purity, by using acetone or alcohol or even inorganic salts like ammonium sulphate or sodium sulphate.
4. Sometimes fractional precipitation of the enzyme is done to obtain it in purest form.
Bacterial α -Amylase:
Bacterial α -amylase is produced by Bacillus subtilis or B. amyloliquefaciens or B. licheniformis. For industrial production of bacterial α -amylase, nowadays submerged culture method is generally employed in many countries.
(a) Preparation of Inoculum:
Pure culture of any of the above-mentioned species of Bacillus is selected as inoculum. Mutants which produce 250 times greater yields than the wild strain are preferred as inoculum.
(b) Preparation of Medium:
The formulation of the production medium and control of fermentation conditions play a major role in the success of enzyme fermentations. The production medium should basically contain an energy source, a carbon source, a nitrogen source and growth requirements such as essential amino acids or vitamins. For obtaining high yields of enzyme, the production medium should also contain certain inducers like lactose.
Sometimes certain compounds like glucose present in the production medium act as repressor for certain enzymes like α-amylase. In such conditions either the concentration of glucose should be kept low or it should be fed intermittently.
The following production medium is generally employed in a submerged culture method (Table 8.5).
(c) Fermentation Process:
The type of fermenter that is used for fungal α-amylase is also used for bacterial α-amylase production. About 1000 to 30,000 gallons of production medium is taken in the fermenter and inoculated. The fermentation is continued upto 4-6 days. The pH of the medium is maintained at 7.0.
Calcium carbonate is used as a buffer for maintaining neutral pH. The temperature is maintained at 30-40°C. The production of α-amylase starts when the bacterial density reaches 109-1010 cells ml-1. However, the enzyme production increases just before the growth rate of the microorganism decreases and spore formation begins.
(d) Harvest and Recovery:
Bacterial α-amylase is harvested and recovered by the same method that is used for the recovery of fungal α-amylase. The most active liquid enzyme preparation contains 2% amylase protein and solid preparation contains 5% amylase proteins. Flow sheet for production of α-amylase is shown in Fig. 8.4.
2. Proteases:
Proteases, second most important industrial enzymes, are produced about 500 tons per year. Proteases are primarily used in detergent, dairy, leather firms, pharmaceutical industries, the manufactured protein hydrolysates, food industry and waste processing.
Organisms:
Proteases are commercially produced both by fungi and bacteria based on optimum pH for their activity. They can be grouped into alkaline, neutral and acid proteases and other groups (table 8.6).
(i) Alkaline Proteases:
Most bacteria and some fungi excrete alkaline proteases. The most important producers of alkaline protease producers are Bacillus licheniformis, B. amyloliquefaciens, B. firmus, B. megaterium and B. pumilus species of Streptomyces such as S. fradiae, S. griseus and S. rectus. Some fungi like Aspergillus niger, A. sojae, A. oryzae, and A. flavus. Proteases of this type have many features of value for use as detergent enzymes. These enzymes are stable at high temperature, at alkaline range of pH (9.0 – 11.0), in association with chelating agents and perborates.
However, their stability is low in the presence of surface active agents resulting in the limited self-life. Subtilisin Carlsberg from B. licheniformis, subtilisin BPN and subtilisin NOVO from B. amyloliqui-faciens are some of the examples of this type of enzymes. These enzymes contain serine at the active site of the molecules and are not inhibited by EDTA (Ethylene diamine tetracetic acid) but are inhibited by DFP (diisopropyl fluorophosphate).
Screening for isolation of microorganisms with better production is done by using strongly basic protein media at pH 10. As most of the wild strains are with low yield and insufficient for industrial utilization, improvement of strain is being done through genetic engineering. Protein engineering has been used to develop modified Bacillus subtilopeptidase with altered amino acid sequences, corresponding changes in enzymatic properties such as substrate specificity, pH optimum and stability to bleaching agents.
Protease production occurs in shaken flasks and small fermenters or in a production fermenter of 40-100 ml capacity at 30-37°C. Production of extracellular proteases is chiefly regulated by the medium composition. The fed-batch process is generally used in order to keep down the concentration of ammonia ions and amino acids which otherwise may repress protease production. High O2 partial pressure is generally necessary for proteases titres, aeration rates are 1 V m-1 and incubation time of 48-72 hrs depending on the organism.
Proteases must be converted into particulate form before they are added to detergents since if dry enzyme powder is inhaled by production workers or users, allergic reaction may result. Enzyme is marketed in a microencapsulated form. To make a suitable encapsulated product, a wet paste of enzyme is melted at 50-70°C with hydrophobic substance such as polyethyl- eneglycol and then converted into tiny particles. These solidified spherical particles are not hazardous when added directly to the detergent. Immobilization in fibrous polymer is another development.
(ii) Neutral Proteases:
These proteases are excreted by Bacillus subtilis, B. cereus, B. megaterium, Pseudomonas aeruginosa, Streptomyces griseus, Aspergillus oryzae, A. sojae and Pyricularia oryzae.
Neutral proteases are relatively unstable.
Calcium and sodium chloride must be added for maximal stability. The pH range of activity is narrow and unstable to increased temperature. These are also quickly inactivated by alkaline proteases which is probably the reason for their restricted industrial application. However, they are used in leather industry and in food industry for manufacture of crackers, bread and rolls.
(iii) Acid Proteases:
These include renin like proteases from fungi which are chiefly used in cheese production. These enzymes have optimum pH 2-4 and used in medicine, in the digestion of soya protein for soya sauce production and to breakdown wheat gluten in the bakery industry.
(iv) Renin:
Milk coagulating enzyme in fourth chamber of 3-4 weeks old calves stomach which is being exploited in cheese production. Due to increased production of cheese and decline in the number of slaughtered calves intensive research has been carried out since 1960 to develop rennin products of microbial origin. A variety of fungi and bacteria have been isolated as producers.
It has also been examined for required characteristics.
Alcaligenes, Bacillus, Corynebacterium, Lactobacillus, Pseudomonas, Serratia and Streptococcus are some of the bacteria that have exhibited potential of rennin production. Similarly species of Aspergillus, Candida, Coriolus, Endothia, Entomophthora, Irpex, Mucor, Penicillium, Rhizopus, Sclerotium and Torulopsis are also reported to produce rennin.
Mucor pusilus var. lindt for solid substrate culture, while Endothia parasitica and Mucor miehei produce this enzyme in submerged culture. Rennin produced by Endothia parastica was first rennin marketed commercially in 1967. The enzyme is an acid protease stable at pH 4.0-5.5 with a molecular weight of 34,000 – 37,500 daltons and is produced in a medium containing 3% soyameal, 1% glucose, 1% skim milk, 0.3% NaNO3, 0.05% K2HPO4,0.025%, MgSO4.7H2O, pH 6.8, at 25°C incubation temperature. At the end of 48 hrs incubation period, mycelium is separated and broth which contains enzyme is concentrated and precipitated in an evaporation process.
Similarly Mucor miehei also produces rennin in a medium containing potato starch 4%, soyameal 3%, ground barley 10%, CaCO3 0.5%, pH 5.5, 5-6 d incubation period at an incubation temperature of 40°C. Microbial renins are stable at high temperature and remain active in the curd after precipitation and subsequently causing harmful proteolysis. Therefore, complementary cDNA from calf renin is transferred into E. coli making possible the first commercial production of the calf rennin by a microorganism.
Applications:
Proteases are employed in variety of fields such as:
1. Biological detergents — alkaline protease
2. Baking—dough modification/gluten weakening and flour improvement
3. Beer brewing —chill proofing of beer to remove protein haze
4. Leather bailing and tendering
5. Cheese manufacture — clotting of milk protein and promotion of ripening aspartic protease, calf chymopsin, aminopeptidase etc.
6. Meat tenderization and removal of meat from bones
7. Flavour control and production in food products
8. Waste treatment- treatment of silver from spent photographic film.
3. Pectinases:
Pectinase is an enzyme complex which contains atleast six enzymes splitting pectins at different sites of the molecule. The basic structure of pectin is α 1,4 linked galacturanic acid with upto 95% its carboxyl groups esterified with methanol. Pectinases are classified according to their attack on the point of molecule (table 8.7).
The methyl ester is split by pectinesterase and the glycosidic bonds of pectin or pectic acid, and chain are split by hydrolysis with endo- polygalacturonase or exo-polygalacturonase. Another method of splitting is transelimination by means of exo-pectate lyase or endopectate lyase or exo-and endo-pectin lyase.
Commercial production of pectinases is carried out by using Aspergillus niger or A. wentii and also species of Rhizopus either as surface or submerged fermentation in a medium containing 2% sucrose and 2% pectin in a fed batch system. The pH is adjusted to 3.0 to 4.0 and incubation temperature 37°C and incubation period upto 60-80 hrs. The enzyme is purified by separating mycelium, by filtration or centrifugation, stabilizing agents are added. The enzyme is precipitated with organic solvents and crude protein is dried and used as enzyme.
Applications:
Pectinases are used primarily for clarification of fruit juices, for maceration of vegetable and fruits, for extraction of oil. By treating with pectinase, the yield of fruit juice during pressing is considerably increased. These are also used in wine classification and coffee bean fermentation.
4. Lipases:
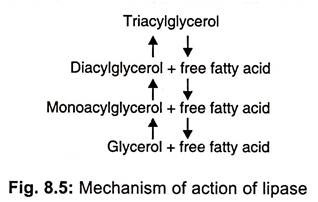
Lipases (glycerolesterhydrolases) split fats (glycerolesters) into di-or monoglycerides and fatty acids (Fig 8.5).
These are usually extracellular. Most of lipases are produced adaptively in the presence of oils and fats. However, some organisms like Penicillium roqueforti are reported to secrete lipase constitutively and in the presence of fats its enzymes production is repressed. Species of Aspergillus, Mucor, Rhizopus, Penicillium, Geotrichum and yeasts (Torulopsis and Candida) are reported to be good producers.
Similarly species of Pseudomonas, Achromobacter and Staphylococcus are good producers of lipases. Lipases are generally bound to the cells and hence inhibit on over production, but addition of cation such as magnesium ion liberates the lipase and leads to a higher enzyme titer in the production process.
Applications:
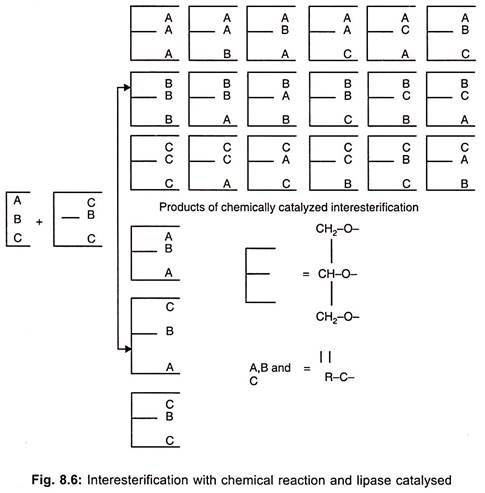
Lipases are primarily marketed for therapeutic purpose as digestive enzymes to supplement pancreatic lipases. These enzymes are used in dairy industry. The cheese ripening process involves lipases. Lipases from Candida cylindraceae is used to hydrolyse oil in soap industry. These are useful in the synthesis of esters from acids and alcohols in non-aqueous medium. Products of interesterification catalyzed by α 1, 3 – regiospecific lipase are illustrated in Fig. 8.6.
These are also useful in improvement of fat quality by inter-esterification (exchange of one fatty acid by another in ester bond) lipases are useful in removal of fat in leather processing. These enzymes are also useful in production of flavour compounds and acceleration of ripening in dairy and meat products.
5. Cellulases:
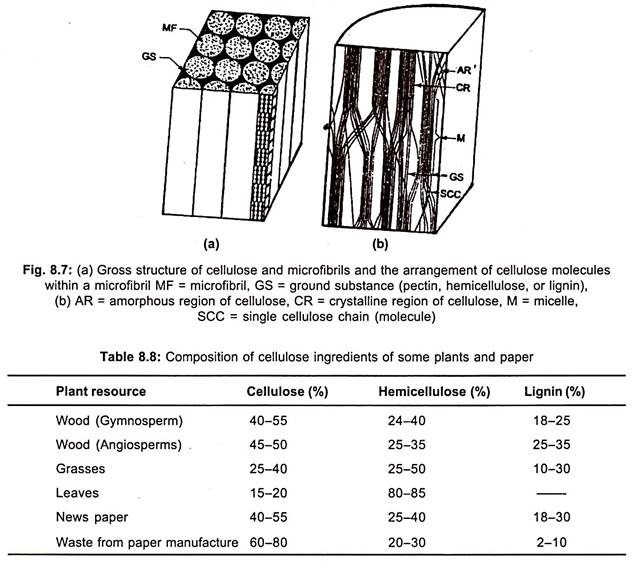
Cellulases are the enzymes which degrade cellulose into fermentable sugars. Although cellulose is available abundantly on the earth in the form of natural plant resources like fallen leaves, flowers, fruits, broken stems, branches and waste wood, it is mixed with substances such as lignin, hemicellulose, starch, proteins etc. (Table 8.8, Fig. 8.7).
All these material are to be treated physically or chemically to breakdown cellulose into fermentable sugars. This breakdown process is called as pretreatment. The pretreatment may be done either chemically or enzymatically. In the second process cellulose enzymes are used to breakdown cellulose into simpler fermentable sugars (Table 8.9).
Most of these enzymes are excreted out by certain microorganisms which include both bacteria and fungi. Such enzymes are called extracellular enzymes. The bacteria include actinomycetes and Cellulomonas and fungi include species of Trichoderma, Penicillium, Thermoascus, Sporotrichum and Humicola. However, for commercial production of cellulases Trichoderma viride, T. reesei, T. koningii, Penicillium funiculosum, P. enersonii, Aureobasidium pullulans, Sporotrichum and pulverulenium are generally employed.
Cellulase is an enzyme complex containing at least three enzymes (table 8.9 and Fig. 8.8):
1. Exo- β-1, 4 glucanase, also called as cellobiodhydrolase or C.
2. Endo-β 1, 4-glucanase also called as carboxymethyl cellulase or endocellulase (Cx).
3. β-1, 4 glucosidase or cellobiase.
Fermentation Production:
The fungus Trichoderma viride is usually used for the commercial production of cellulose. Inoculum of the fungus is raised from pure or mutant culture of Trichoderma viride GM 9414 by a process of repeated sub-culturing. Large fermenters of stainless steel are employed for fermentative production of celluloses.
The production medium consists of the following ingredients:
The fermentation is carried out at 30°C for about 140 hours. Maximum fungal growth occurs under these conditions resulting in the maximum yield of the cellulose. However, the yield of enzyme is dependent upon the cellulose concentration (Fig. 8.6). After the completion of the fermentative process the products recovered from the fermenter and the fungal mycelium is separated by filtration. Afterwards purification of the enzyme was carried out using ion exchangers.
Applications:
1. Fruit juice and olive production and processing.
2. Wine and beer production and processing.
3. Malting-speedy modification of grains.
4. Textile processing-bio polishing of cellulose fibers.
5. Wood pulp processing.
6. Glucose Isomerase:
Glucose isomerase causes the isomerization of glucose to fructose. The reaction is reversible and a mixture of glucose and fructose is produced. The ratio of these products depends on the enzyme used and reaction conditions such as incubation time, pH and temperature. The enzyme has become of commercial value because price of sugar has increased as compared to that of starch.
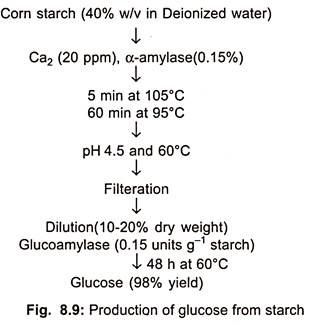
In U.S. about 4 × 106 tons of isosyrup is being produced, while in Germany 10,000-12,000 tons per year. Starch can be converted to glucose by either acid hydrolysis or enzyme hydrolysis (Fig. 8.9).
The advantage of starch hydrolysis over direct sugar production (sugar beets) is that initial materials used such as wheat, corn, cassava and to some extent potatoes are non- perishable, but sugar beets are available only 100 days per year.
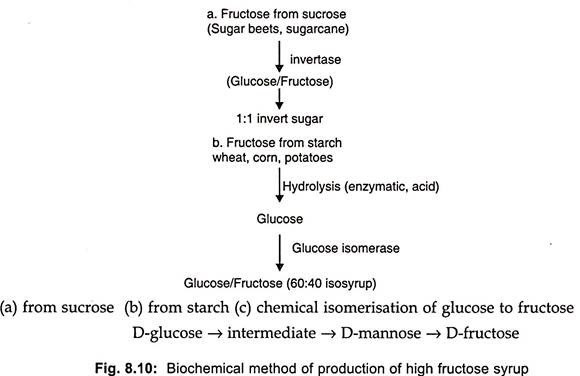
Glucose has 70-75%, the sweetening strength of beet sugar (sucrose) but β-D-fructose pyranose (fructose), the sweetest monosaccharide has twice the sweetening strength of sucrose. Thus, processes for the manufacture of fructose are of considerable value. Fructose can be produced biochemically from sucrose or starch by isomerase, glucose production (Fig. 8.10 a and b).
Fructose can also be produced chemically from glucose at high temperature under alkaline conditions but byproducts are formed such as psicose which cannot be metabolized in the human body. Thus chemical production of fructose is not acceptable to the food industry.
Fermentative Production:
Glucose isomerase is used in production of high fructose syrup (HFCs) or isosyrup must fulfill the following criteria:
1. Low pH optimum to avoid side reactions.
2. High specific activity.
3. High temperature optima.
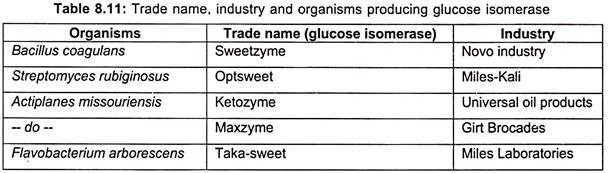
Some of the microorganisms producing glucose isomerase are listed in table 8.10.
Practical conversion yields are in the range of 40-50% where fructose concentration can be increased to 55% by chromatographic enrichment. Alternatively the fructose can now be separated from glucose (+) fructose mixture and the remaining glucose fraction isomerzed to produce a final combined product containing upto 80% fructose. HFCs from corn starch is cheaper than from sucrose but have same intensity of sweetening.
Consequently they have become major food ingredients particularly in North America and annual production of HFCs is now in excess of 8 × 109 kg. Some of the bacteria such as Escherichia intermedia, E. freudii and Aerobacter aerogenes can produce isomerase. Though they are able to produce glucose isomerase which is variant and requires arsenium for its activity, hence it cannot be used in food production.
The commercial process for production of fructose (Fig. 8.11) from glucose became feasible only when procedures for immobilization of the enzyme were developed (Fig. 8.12).
Since, glucose isomerase is formed intracellularly in most strains; many commercial processes are carried out with immobilized cells or by addition of partly broken cells. Commercial production of glucose isomerase along with producing organism and industry are listed in table 8.11.