In this article we will discuss about Electrophoresis:- 1. Meaning of Electrophoresis 2. Definition of Electrophoresis 3. Classification.
Meaning of Electrophoresis:
The term electrophoresis describes the migration of a charged particle under the influence of electric field (electro-charged particle and phoresis-movement). Many important biological molecules such as amino acids, peptides, proteins, nucleotides, nucleic acids possess ionisable groups and, therefore, at any given pH, exists in solution as electrically charged species either as cations or anions.
Under the charge of an electric field these charged particles will migrate either to cathode or to anode, depending on the nature of their net charge. This is one of the most fundamental processes used in all types of molecular biology and RDT experiments.
Definition of Electrophoresis:
Electrophoresis is migration of charged particles or molecules in a medium under the influence of an applied electric field.
The Rate of migration of charged molecules depends upon following factors:
(a) The strength of electric field, size and shape.
(b) Relative hydrophobicity of the sample.
(c) Ionic strength and temperature of the buffer.
(d) Molecular size of the taken biomolecule.
(e) Net charge density of the taken bio molecule.
(f) Shape of the taken biomolecule.
In the process of electrophoresis large molecules have more difficulty in moving through the supporting medium (i.e., gel) whereas the smaller medium has more mobility through it.
Classification of Electrophoresis:
All modern electrophoretic apparatus have supporting media these days. A supporting medium is a physical support through which the charged molecules get separated. It has two primary functions-adsorption and molecular sieving of the taken molecules which are intended to be separated.
Now a question arises why to take a supporting medium and why not to follow the basic principles of electrochemistry, using an electrophoretic tank with two electrodes for separating these charged molecules. This is what had been done in the beginning days.
The pioneering work on electrophoresis by Tiselieus et al. in the year 1948 was performed in free solution only. However, it was soon realized that many of the problems associated with this approach, particularly the adverse effects of convection currents (Current caused by the expansion of a liquid, solid, or gas as its temperature rises.
This makes the separated molecules distorted and very randomly arranged), could be minimized by stabilizing the medium. This was achieved by carrying out electrophoresis on a porous mechanical support. Due to its viscosity (which is absent in free solution) the supporting media cut down the convention currents due to which we get the separated molecules in sharp zones.
Some of the frequently used supporting media are starch, agar, polyacrylamide and agarose. Depending upon the presence or absence of supporting media the electrophoresis can be classified as free electrophoresis and zone electrophoresis.
A. Free Electrophoresis:
In this type of electrophoresis a free electrolyte is taken in place of supporting media. Nowadays this type of electrophoresis has become out-dated and mostly used in non-biological experiments.
It is mostly of two types―the micro-electrophoresis which is mostly used in calculation of Zeta potentials (a colloidal property of cells in a liquid medium) of the cells and moving boundary electrophoresis which for many years had been used for quantitative analysis of complex mixtures of macromolecules, especially proteins.
B. Zone Electrophoresis:
This is the most prevalent electrophoretic technique of these days. In this type of electrophoresis the separation process is carried out on a stabilizing media as discussed above.
The zone electrophoresis is of following types;
(a) Paper electrophoresis
(b) Cellulose acetate electrophoresis
(c) Capillary electrophoresis
(d) Gel electrophoresis – which further includes Agarose gel electrophoresis, SDS PAGE, PFGE and two-dimensional electrophoresis.
C. Paper Electrophoresis:
In this type of electrophoresis a filter paper (like chromatography paper) having slight adsorption capacity and uniform pore size is used as the supporting medium for separation of samples under the influence of an applied electric field. While carrying out paper electrophoresis, a strip of filter paper is moistened with buffer and ends of the strip are immersed into buffer reservoirs containing the electrodes.
The samples are spotted in the centre of the paper, high voltage is applied, and the spots migrate according to their charges. After electrophoresis, the separated components can be detected by a variety of staining techniques, depending upon their chemical identity.
Applications:
(a) Serum analysis for diagnostic purpose is carried out by paper electrophoresis.
(b) Muscle protein (Myosin), egg protein (albumin), milk protein (casein), snake and insect venoms have been satisfactorily analysed using paper electrophoresis.
Disadvantage:
It is very time-taking. Around 14-16 hours are needed for the process of complete separation.
D. Cellulose Acetate Electrophoresis:
It is a modified version of paper electrophoresis developed by Kohn in 1958. In this type of electrophoresis bacteriological acetate membrane filters are taken in place of regular chromatography paper.
The followings are advantages of cellulose acetate strips over chromatography paper:
(a) The cellulose acetate strips are chemically pure and free of lignin and hemicelluloses and generally act as barriers in free moment of large molecules.
(b) Because of low content of glucose cellulose acetate strips Eire suitable for electrophoresis of polysaccharides.
(c) Cellulose acetate is not hydrophilic and this holds very little buffer which further helps for a better resolution in a short time.
Applications:
It is especially used for clinical investigation such as separation of glycoproteins, lipoproteins and haemoglobin from blood.
E. Capillary electrophoresis:
Capillarity of narrow bore tube is employed to separate the samples based on their size: charge ratio. Capillary electrophoresis (CE) is relatively new separation technique compared to the traditional techniques such as agarose gel electrophoresis or SDS-PAGE.
It provides very attractive features which make it both competitive and a good alternative. One of the major advantages of CE over other separation technique is the ability to separate both charged and non-charged molecules. In CE, separation of analyte ions is performed in an electrolyte solution (background electrolyte) present in a narrow fused-silica capillary.
The ends of the capillary are immersed into vials (inlet and outlet) filled with electrolyte solution, which also contain electrodes connected to a high voltage supply (Fig. 3.12). The sample solution is introduced in the capillary as a small plug by applying pressure (hydrodynamic injection) or voltage (electro kinetic injection).
With the application of high voltage (5 – 30 kV) across the capillary, zones of analyte are formed due to different electrophoretic mobilities of ionic species and migrate towards the outlet side of the capillary. In fact, different ions can be separated when their charge/size ratio differs. Before reaching the end of the capillary, the separated analyte bands are detected directly through the capillary wall.
Advantages:
(a) High separation efficiency
(b) Short analysis time
(c) Low sample and electrolyte consumption
(d) Low waste generation
(e) Ease of operation
Disadvantages:
Due to small diameter of the capillary tube, heat is dissipated that causes increased diffusion. Due to this the resolution is not always proper.
Applications:
CE is used in the following analysis of food, pharmaceutical products and environmental pollutants.
F. Gel Electrophoresis:
Gel electrophoresis involves the use of gel as supporting media for separation of DNA, RNA or proteins under the influence of electric charge. It is usually performed for analytical purposes but may be used as a preparative technique to partially purify molecules prior to use for other methods such as mass spectrometry, PCR, cloning, DNA sequencing and immuno-blotting.
This is the most commonly used electrophoresis in biotechnology laboratories and is used for almost all types of experiments in RD.
Principle:
The electromotive force (EMF) generated across the electrodes pushes or pulls the molecules (nucleic acids or proteins) through the gel matrix. The molecules move towards the anode if negatively charged or towards the cathode if positively charged.
A typical gel electrophoresis apparatus is of two kinds:
(a) Vertical Gel Apparatus:
It is used for the separation of proteins in SDS-PAGE.
(b) Horizontal Gel Apparatus:
It is used for immune-electrophoresis, isoelectric focusing and electrophoresis of DNA and RNA in the agarose gel.
Types of Gel-Electrophoresis:
(a) Agarose Gel Electrophoresis:
The supporting medium in this type of electrophoresis is agarose gel. This is used for the electrophoresis of Nucleic acids like DNA and RNA.
Principle:
When a potential difference is applied across the electrodes of a horizontal electrophoretic tank containing agarose gel and biomolecules (such as nucleic acids) are loaded, then they get separated according to their molecular size (bigger molecules have more molecular size and smaller molecules have small molecular size) and move to their respective electrodes. Here the agarose gel acts as a sieve.
As in a sieve the large particles stay above and the particles which are smaller than the pore size passes through it, similarly in the gel the larger and the bulky molecules stay behind whereas the smaller molecules move faster and quickly towards their respective electrodes.
This process may be imagined like a running competition. The one who is thinner and have a flexible body will be at the ending point sooner than the one who is fat and bulky.
Instrumentation:
1. Physical Apparatus:
This includes the physical body of the experimental set-up.
It is of following three types:
(a) Electrophoretic Apparatus:
Horizontal Gel Electrophoresis System is designed for very fast and clear separation of DNA restriction fragments in agarose gels. Gel apparatus vary according to manufacturer. The simplest apparatus is called a flatbed horizontal apparatus. This apparatus consists of two buffer chambers, a wick poured from the agarose, and flat horizontal bed of agarose upon which small wells for sample loading are located.
(b) Power Supply:
For a standard agarose gel electrophoresis a voltage of 5 volts per cm of gel is applied (the cm value is the distance between the two electrodes, not the length of the gel).
(c) Trans illuminator:
This is an ultraviolet light box which is used to visualize ethidium bromide-stained DNA in gels.
2. Chemical Components:
This includes all the chemical components which are involved in the electrophoresis of the biomolecule.
(a) Supporting Media:
Agarose is a polysaccharide extracted from seaweed. It is a linear polymer composed of alternating isomers of the sugar D- and L-galactose. Agarose melts approximately at 90°C and gels approximately at 40°C (Fig. 3.14). This gelation results in a mesh of channels with a diameter varying form 50-200nm. The diameters of these channels determine the final porosity of the gel and acts as the sieve.
By using gels with different concentrations of agarose, one can resolve different sizes of DNA fragments. Higher concentrations of agarose facilitate separation of small DNAs, while low agarose concentration is maintained during the separation of DNA having higher molecular weight. It is typically used at concentrations of 0.5 to 2%.
(b) Buffer:
The gel is immersed within an electrophoresis buffer that provides ions to carry a current and some type of buffer to maintain the pH at a relatively constant value. Depending on the size of the DNA electrophoresed and the application, different buffers can be used for agarose electrophoresis.
TAE buffer (or Tris Acetate EDTA) is the most commonly used agarose gel electrophoresis buffer. TAE has the lowest buffering capacity of the buffers, however, TAE offers the best resolution for larger DNA. TAE also requires a lower voltage and more time.
TBE buffer (Tris/Borate/EDTA) is often used for smaller DNA fragments (i.e., less than 500bp). Sodium borate or SB buffer is a new buffer but it is ineffective for resolving fragments larger than 5 kb. SB has advantages in its low conductivity, allowing higher voltages (up to 35 V/cm). This could allow a shorter analysis time for routine electrophoresis. Two types of buffers are used in the process of agarose electrophoresis.
Electrophoresis Buffer:
This type of buffer is used to bathe the gel placed on horizontal tank. Usually Tris-ace- tate-EDTA (TAE) or Tris-borate- EDTA (TBE) is taken as electrophoresis buffer.
Loading Buffer:
This contains a dense medium (e.g., glycerol) to allow the sample to “fall” into the sample wells, and one or two tracking dyes, which migrate in the gel and allow visual monitoring of the extent of electrophoresis.
(c) Dye:
This is a tracking agent that aims at visualizing the separated nucleic acid after the process of electrophoresis. In the agarose gel electrophoresis Ethidium bromide is used as a dye. Ethidium bromide is a fluorescent dye that binds to DNA and intercalates between the stacked bases.
When irradiated with UV light of a wavelength of 302 nm, ethidium bromide will emit fluorescence light of a wavelength of 590 nm (orange). After the process of electrophoresis the gel is observed under the beam of UV light produced by the trans-illuminator. The dye is added to the gel during its preparation only.
Procedure:
First of all agarose powder is mixed with electrophoresis buffer to the desired concentration, then heated in a microwave oven until completely melted. Most commonly, ethidium bromide is added to the gel (final concentration 0.5 mg/ml) at this point to facilitate visualization of DNA after electrophoresis.
After cooling the solution to about 60°C, it is poured into a casting tray containing a sample comb and allowed to solidify at room temperature. After the gel has solidified, the comb is removed, using care not to rip the bottom of the wells.
The gel, still in its plastic tray, is inserted horizontally into the electrophoresis chamber and just covered with buffer, Samples containing DNA mixed with loading buffer are then pipetted into the sample wells, the lid and power leads are placed on the apparatus, and a current is applied.
You should be confirmed that current is flowing by observing bubbles coming off the electrodes. DNA will migrate towards the positive electrode, which is usually coloured red. When adequate migration has occurred, DNA fragments are visualized by staining with ethidium bromide.
This fluorescent dye intercalates between bases of DNA and RNA. It is often incorporated into the gel so that staining occurs during electrophoresis, but the gel can also be stained after electrophoresis by soaking in a dilute solution of ethidium bromide.
To visualize DNA or RNA, the gel is placed on a ultraviolet trans-illuminator. Be aware that DNA will diffuse within the gel over time, and examination or photography should take place shortly after cessation of electrophoresis.
Application of Agarose Gel Electrophoresis:
1. Separation of restriction enzyme digested DNA including genomic DNA, prior to Southern Blot transfer. It is often used for separating RNA prior to Northern transfer.
2. Analysis of PCR products after polymerase chain reaction to assess for target DNA amplification.
3. Allowing estimation of the size of DNA molecules using a DNA marker or ladder which contains DNA fragments of various known sizes.
4. Allows the rough estimation of DNA quantity and quality.
5. Quantity is assessed using lambda DNA ladder which contains specific amounts of DNA in different bands.
6. Quality of DNA is assessed by observing the absence of streaking or fragments (or contaminating DNA bands).
7. Other techniques rely on agarose gel electrophoresis for DNA separation including DNA fingerprinting.
Advantages and Disadvantages of Agarose Gel Electrophoresis:
The advantages are that the gel is easily poured, and does not denature the samples. The samples can also be recovered.
The disadvantages are that gels can melt during electrophoresis, the buffer can become exhausted, and different forms of genetic material may run in unpredictable forms.
(B) SDS-Page:
Sodium Dodecyl Sulfate (SDS) polyacrylamide gel electrophoresis is mostly used to separate proteins accordingly by size. This is one of the most powerful techniques to separate proteins on the basis of their molecular weight.
Principle:
This technique uses anionic detergent Sodium Dodecyl Sulfate (SDS) which dissociates proteins into their individual polypeptide subunits and gives a uniform negative charge along each denatured polypeptide. SDS also performs another important task.
It forces polypeptides to extend their conformations to achieve similar charge: mass ratio. SDS treatment therefore eliminates the effects of differences in shape so that chain length, which reflects their molecular mass, is the sole determinant of migration rate of proteins in the process of electrophoresis.
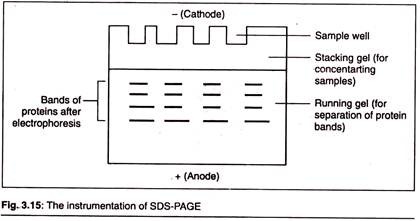
When these denatured polypeptides are loaded at the cathode end of an electrophoretic tank having polyacrylamide gel (as the supporting media) and subjected to an electric field, then we get clear bands of proteins arranged in an decreasing order of their molecular mass from the cathode to anode.
The rate of movement is influenced by the gel’s pore size and the strength of electric field. In SDS- PAGE the vertical gel apparatus is mostly used. Although it is used to separate proteins on a routine basis, SDS-PAGE can also be used to separate DNA and RNA molecules.
Instrumentation:
Physical Apparatus:
This includes the physical body of the experimental set-up. It is of following two types:
a. Electrophoretic Apparatus:
Vertical horizontal tank with electrodes, gel cassettes, Teflon spacers, clips, pipette or syringe, comb, acrylic cover.
b. Power Supply:
A power supply of 100-200 volts is needed. This is ideal for running and transferring protein resolving gels.
c. Staining Box:
These are trays in which the gels are stained and made up of clear plastics. These are resistant to most organic dyes, silver and other stains.
Chemical Components:
This includes the following:
(a) Supporting Media:
SDS-PAGE acrylamide is used as the supporting medium. It is a white crystalline powder, when acrylamide dissolves in water, it undergoes polymerization reaction to form a net-like structure called polyacrylamide. Polyacrylamide is a polymer (CF2CHCONH2-) formed from acrylamide subunits that can also be readily cross-linked.
This type of electrophoresis has a discontinuous system of gel, i.e., we have two different systems of gels present in the electrophoretic tank physically placed one over another.
These are as follows:
Resolving Gel:
This is also called separating or running gel. The separating gel constitutes about 2/3rd of the length of gel plate and is prepared by 5-10% of acrylamide. The pores in this gel (which is formed after the polyacrylamide is cross- linked) are numerous and smaller in diameter which impacts sieving property to this gel.
Stacking gel:
Stacking gel is poured on the top of resolving gel and a gel comb is inserted which forms the well. It is the upper layer of gel and constitutes 1/3rd of the gel plate. The percentage of acrylamide is chosen depending on the size of protein that one wishes to identify or probe in the sample.
The smaller the known weight, the higher the percentage that should be used. Generally, the percentage of acrylamide in stacking gel is 2-5%. It is highly porous and devoid of molecular sieving action.
(b) Buffer:
Two types of buffers are used in SDS-PAGE. The lower reservoir (which has the running gel) has amine buffers. It is adjusted by using HCl. The upper reservoir (which has stacking gel) also has amine buffers but its pH is slightly above that of running gel buffer and is adjusted with glycine instead of HCl.
(c) Dissociating Agent:
SDS is the most common dissociating agent used to denature native proteins to individual polypeptides. When a protein mixture is heated to 100°C in presence of SDS, the detergent wraps around the polypeptide backbone.
It binds to polypeptides in a constant weight ratio of 1.4 g/g of polypeptide. In this process, the intrinsic charges of polypeptides becomes negligible when compared to the negative charges contributed by SDS. Thus, polypeptides after treatment become rod-like structure possessing uniform charge density, that is the same net negative charge per unit length.
(d) Stains:
The stains are used to see the bands of separated proteins after the process of electrophoresis. Coomassie Brilliant Blue R-250 (CBB) is the most popular protein stain. It is an anionic dye, which binds with proteins non-specifically.
Proteins in the gel are fixed by acetic acid and simultaneously stained. The excess dye incorporated in the gel can be removed by de-staining with the same solution but without the dye. The proteins are detected as blue bands on a clear background.
The solution of proteins to be analysed is first mixed with SDS, an anionic detergent, an anionic detergent which denatures secondary structure. Besides addition of SDS, proteins may optionally be boiled in the presence of a reducing agent, such as Di-Thio-Threitol (DTT) or 2-mercaptoethanol, which further denatures the proteins by reducing disulfide linkages, thus overcoming some forms of tertiary protein folding, and breaking up quaternary protein structure (Oligomeric subunits).
This is known as reducing SDS-PAGE, and is most commonly used. Non-reducing SDS-PAGE (no boiling and no reducing agent) may be used when native structure is important in further analysis (e.g., enzyme activity, shown by the use of zymograms). The denatured proteins are subsequently loaded into the wells of stacking gel flooded with stacking buffer.
This end is connected with the cathode of power supply. Then an electric current is applied across the gel, causing negatively charged proteins to migrate across the gel towards anode. After crossing the stacking gel, denatured proteins enter the running gel which has its own buffer system (running buffer).
Depending on their size, each protein will move differently through the gel matrix: short proteins will more easily fit through the pores in the gel, while larger ones will have more difficulty.
After the separation is over the gel is gently taken out and transferred to the staining box and treated with the staining dye, e.g., CBB R-250. Excess of stains are removed by de-staining using acetic acid solution. The bands appear to be blue stained which are then analysed according to the need of the experiment.
Application:
SDS-PAGE has many applications. It is mostly used for following purposes:
1. Establishing protein size
2. Protein identification
3. Determining sample purity
4. Identifying disulfide bonds
5. Quantifying proteins
6. Blotting applications
Advantages of SDS-PAGE:
SDS-PAGE has following advantages:
1. Mobility of the molecules is high and separation is rapid.
2. All the proteins are negatively charged; therefore, all migrate towards anode.
3. The proteins treated with SDS fixed dyes are better than the native proteins.
4. SDS solubilizes all proteins, including very hydrophobic and even denatured proteins.
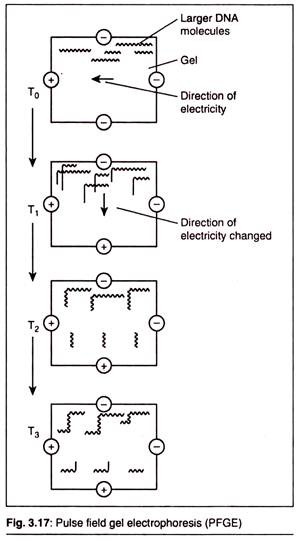
(C) Pulse Field Gel Electrophoresis (PFGE):
Conventional methods of gel electrophoresis are carried out by placing DNA samples in a solid matrix (agarose or polyacrylamide) and inducing the molecules to migrate through the gel under a static electric field. When DNA molecules are under the influence of this electric field, they elongate and align themselves with the field.
In DNA electrophoresis by the standard method, however, DNA molecules which are larger than 20kb cannot be separated either by agarose gel electrophoresis or by SDS-PAGE. This is done by pulse field gel electrophoresis.
In this technique the periodic changing of orientation of the electric field is carried out. This forces the large DNA molecules in the gel to relax upon removal of the first and elongate to align with the new field.
(D) Two-Dimensional Electrophoresis:
This is a very sensitive technique to purify a mixture of polypeptides.
It is carried out in two dimensions:
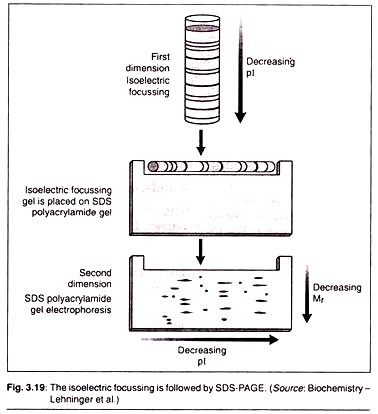
1st Dimension Separation:
It is where we carry out a process called isoelectric focusing. The net charge of any protein is the sum total of all positive and negative charges in it. These charges are determined by ionizable acidic and basic side chains and prosthetic groups of the proteins.
The isolectric point (pi) of a polypeptide is that pH value at which its net charge is zero. At this point the polypeptides cannot move anywhere in the gel and makes bands there. Isoelectric focusing is a procedure used to determine the isoelectric point (pi) of a protein (Fig. 3.18).
A pH gradient is established by allowing a mixture of low molecular weight organic acids and bases called ampholytes to distribute themselves in an electric field generated across the gel. When a protein mixture is applied, each protein migrates until it reaches the pH that matches its pi. Proteins with different isoelectric points are thus distributed differently throughout the gel.
2nd Dimension Separation:
Isoelectric focusing is followed by SDS-PAGE as discussed above. In this process the proteins will move from cathode to anode in the decreasing order of their molecular mass (Mr).