In this article we will discuss about the southern, northern and western blotting of DNA.
Southern Blotting of DNA:
DNA fragments on agarose gels give no indication of function or sequence. Blotting is a technique used to identify specific DNA sequences. Usually, larger fragments of DNA (genomic DNA) are made into conveniently sized fragments by restriction analysis.
Then they are run through an agarose gel. The separated fragments in the gel are transferred to a nylon membrane by capillary transfer or vacuum blotting. The sequences of interest can be identified by hybridization using radioactive or non-radioactive probes and visualized by autoradiography or staining.
1. Destain the agarose gel in sufficient amount of water with shaking under room temperature.
2. Carefully transfer the gel to 0.2 M HCl and shake for 10 minutes at room temperature. (DNA fragments larger than 10 kb do not transfer to blotting membrane efficiently. In order to facilitate their transfer, these fragments are reduced in size, either by depurination or UV irradiation. During this period the colour of the marker dye (bromophenol blue) will change from blue to yellow, indicating that the gel has been completely saturated with the acid. This depurination step should not be too long, since small fragments attach less firmly with the membrane. Depurination is recommended only for fragments larger than 10 kb or otherwise these yield fuzzy bands or smears in the final autoradiograph, presumably because of increased diffusion of DNA during transfer.)
3. Denature the double stranded DNA in order to create suitable hybridization targets by incubating the gel with denaturation buffer containing 0.5 M NaOH and 1.5 M NaCl for 15-30 minutes with gentle shaking.
(During denaturation the bromophenol blue will regain its colour.)
4. Incubate the gel with neutralization solution containing 1.5 M NaCl and 0.5 M Tris-HCl pH 7.5 for 30 minutes.
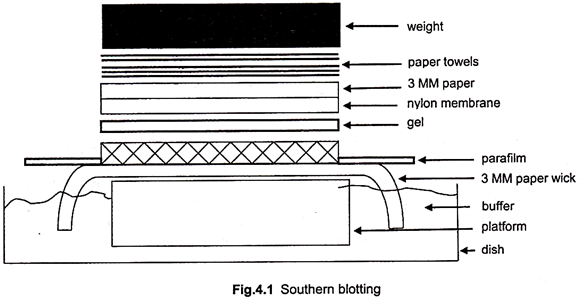
5. Set up a Southern transfer as in Fig.4.1.
6. Place the gel on the platform. Remove any air bubbles trapped between the gel and the platform by rolling a pipette several times back and forth over the gel.
7. Surround the gel with plasticwrap. This ensures that the transfer buffer (10X SSC) moves only through the gel and not around it.
8. Place the blotting membrane (nylon membrane) on the top of the gel so that it covers the entire surface. Remove any air bubbles between the membrane and gel.
9. Place two precut sheets of Whatmann 3 MM paper in 10X SSC, and place them on the top of the nylon membrane. Again remove any trapped air bubbles.
10. Place a 15-20 cm stack of dry paper towels on top of the filter paper.
(Transfer efficiency is improved by removing the wet paper towels and replacing them with dry papers at least once during transfer.)
11. Place a glass plate on the top of the paper towels. Place a weight (about 500 g) on top of the plate.
12. Allow the transfer for 12-18 hours.
Fixing the DNA:
13. Immediately after transfer, remove the weight, paper towels and filter papers. Turn over the gel and blotting membrane together (gel side on the top) on a filter paper towel. Mark the positions of gel lanes on the membrane using a ball point pen. Remove carefully the membrane from the gel.
14. Fix the DNA to the blot, either by baking or UV cross linking.
(UV cross-linking generally gives better results. To bake, first let the blot dry on a sheet of filter paper, then place between sheets of filter papers, and bake it at 80°C. To UV cross link protect the surface of the DNA side of the membrane with plasticwrap and then expose this side to the UV source for 3 minutes.)
15. Preserve the blot if not used immediately at room temperature covered in plasticwrap.
Northern Blotting of DNA:
After separating RNA molecules in a denatured gel, RNA molecules in the gel are transferred to nylon or nitrocellulose membrane by capillary transfer or vacuum blotting. Since DNA blotting is commonly referred as ‘Southern blotting’, after its inventor E M Southern, the RNA blotting is called ‘northern blotting’.
Northern blotting is generally carried out using positively charged nylon membranes because of their greater strength and easy handling. It is always recommended to handle the membranes with gloves.
Preparation of Gel:
1. After gel electrophoresis, incubate the gel in 200 ml of RNase free water and then in 200 ml of 0.05 M NaOH for 15 minutes.
2. Incubate the gel for 10 minutes in 200 ml of 10X SSC to neutralize the NaOH.
3. Prepare a northern blotting set up for Southern blotting (step 5 to step 12 of Southern blotting protocol).
The gel contains formaldehyde, which will diffuse out of the gel during transfer. Formaldehyde is toxic. Perform the transfer in a fume hood to avoid inhalation. Wear gloves and take appropriate safety precautions when handling.
4. Wash the nylon membrane for 1 minute in 100-200 ml 10X SSC.
5. Fix the RNA to the blot by baking or UV cross-linking (as followed for Southern blotting).
6. Preserve the blot if not used immediately at 4°C covered in plasticwrap.
Western Blotting (Immunoblotting) of DNA:
Immunoblotting or protein blotting involves transferring protein bands from an acrylamide gel to a more stable and immobilizing medium such as nitrocellulose membrane. Once transferred, detection of specific proteins with antibodies can be possible. The approach is similar to the method used to transfer DNA from agarose gels to nylon membrane (Southern blotting).
Protocol:
1. Wash the unstained protein gel following electrophoresis (SDS-PAGE) in distilled water.
2. Transfer the gel to transfer buffer for 15-30 minutes.
3. Cut pieces of Whatmann filter paper No.3 and nitrocellulose sheet to the size of the acrylamide gel. Immerse them in distilled water and soak in transfer buffer for 15-30 minutes.
(Whatmann filter papers are used as supporting material. Care should be taken while handling nitrocellulose membrane, and avoid touching the paper as finger prints lead to unbinding of peptides during transfer).
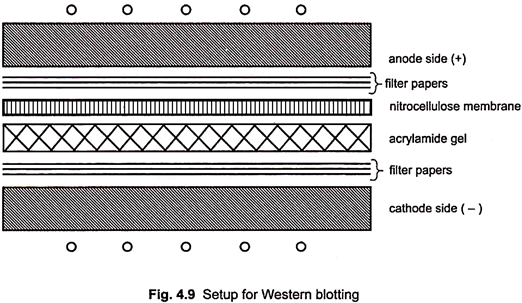
4. Assemble a sandwich as follows –
i. Place the wet filter paper on the negative side of the blotter.
ii. Pour transfer buffer over the filter paper little by little.
iii. Remove any air bubbles by rolling pipette over the paper.
iv. Place the gel on the paper.
v. Put transfer buffer over the gel and remove air bubbles.
vi. Place the nitrocellulose membrane on the gel and remove air bubbles.
vii. Wet the 3 MM filter paper and place it over the nitrocellulose sheet on the positive side of the blotter.
viii. Pour transfer buffer over the filter paper and remove air bubbles.
ix. Place the positive side of the blotter over the filter paper.
x. Put rubber bands around the sandwich.
5. If it is a semi-dry blotting apparatus, run at 60 volts constantly for 2- 6 hours (for mini gel) at 4°C (follow manufacturer’s instruction).
6. If it is a wet blotting apparatus, keep the sandwich in the blotting tank containing transfer buffer and run at 60 volts constantly for 2-4 hours (for mini gel) at room temperature (follow manufacturer’s instruction).
7. After transfer remove the nitrocellulose membrane and air dry it.
8. Soak the membrane (blot) in a blocking solution (Tris buffered saline, TBS) containing milk powder, preferably 5% milk powder for 3 hours with shaking.
9. Wash the blot 2-3 times with TBS-Tween 20 for 10 minutes with shaking.
10. Incubate the blot with primary antibody (primary antibody dilution factor can be estimated by ELISA) in 1:1000 dilutions with TBS in 3% milk powder for 2-3 hours under shaking.
11. Wash 2-3 times with TBS-Tween 20, each 10 minutes with shaking.
12. Transfer the blot to secondary antibody (anti-rabbit IgG) in 1:500 dilutions (follow the company’s recommendation) with TBS-Tween 20 and 3% milk powder for 1 hour under shaking.
13. Wash two times in TBS-Tween 20 and two times in TBS alone.
14. Develop the colour by adding substrate solution.
15. Stop the reaction by transferring the blot to distilled water.
Preparation of Solutions:
Transfer Buffer (1000 ml):
Tris – 3 g
Glycine – 14.4 g
Distilled water – 800 ml
Adjust the volume to 1000 ml with methanol.
Blocking Solution (TBS) (100 ml):
Dissolve 5 g milk powder in 10 mM Tris-HCl, pH 7.5.
Add 900 mg (0.9%) NaCl and 50 µl Tween 20.
Substrate Solution (50 ml):
Dissolve 30 mg 4-chloro-1-naphthol in 10 ml of methanol.
Add 30 µl H2O2 in 40 ml of TBS.
Mix these solutions thoroughly and add to the blot (nitrocellulose membrane).
Primary Antibody:
1: 1000 dilutions. Use 100 µl for 100 ml. Do ELISA for determining the dilution factor.
Secondary Antibody:
1:500 dilutions (use 200 µl for 100 ml). Follow the company recommended dilution factor.
Enzyme Linked Immunosorbant Assay (ELISA):
This is a method analogous to the immunodetection of proteins on a membrane. It is used for the quantitative assay of proteins in solution. In this technique, proteins are immobilized on a solid support (on the 96 well ELISA plate) and used to capture molecules that bind to the protein being assayed.
After a wash step to remove any non-specific bound material, a secondary antibody (specific for the protein being assayed) is added. This secondary antibody is usually conjugated to an enzyme that allows its detection by chromogenic or chemiluminescent methods.
In one method, an antibody that binds an epitope on a target protein is immobilized, and a test solution added. The immobilized antibody will capture any target protein present in the sample. A wash step removes non-specifically bound material, and subsequently a second antibody is added that reacts with a second epitope on the protein.
In the other method, a protein can be immobilized on a solid support, and antibodies reacting with the protein can be detected and quantified in a test solution by addition of a secondary antibody that reacts with the primary antibody.
Protocol:
1. Make a serial dilution of the protein to be immobilized in coating buffers.
2. Add 200 µl of the protein solution to each well, and incubate overnight at 4°C.
3. Wash the wells 4 times with PBS each time for 10-20 seconds and dry the wells by tapping the plate on a paper towel.
4. Block wells with 250 µl of blocking buffer for 2 hours at room temperature (approximately 25°C) on a shaker.
5. Wash the wells 4 times with PBS each time for 1 minute and dry the wells by tapping the plate on a paper towel.
6. Add 200 µl of anti-target protein antibody (primary antibody) diluted 1/2000 in PBS containing BSA. Cover the plates and incubate for 1-2 hours at room temperature (Incubating overnight at 4°C can increase antibody sensitivity).
7. Wash the wells 4 times with PBS-Tween 20 each time for 10-60 seconds and dry the wells by gently tapping on paper towel after the wash.
8. Dilute secondary antibody in PBS containing BSA according to the Company recommendations. Add 200 µl of the diluted secondary antibody to each well and incubate at room temperature for 1 hour.
9. Wash the wells 4 times with PBS-Tween 20, each time 10-60 seconds and dry the wells by gently tapping the plate on paper towel.
10. Add 200 µl of substrate solution and monitor the colour development in a microplate reader.
11. Stop the reaction after a period of 45 minutes by adding 50 µl of stop solution.
12. Read absorption in ELISA reader at 416 nm (for HRP system) and 405 nm (for AP system).
Preparation of Solutions:
Phosphate Buffered Saline (PBS) (100 ml):
50 mM K3PO4 – 71.7 ml of 0.5 MK3PO4
50 mM KH2PO4 – 28.3 ml of 0.5 M KH2PO4
150 mM NaCl – 8.8 g
The pH should be 7.2 without adjustment.
Coating Buffers:
A. 50 mM Sodium Carbonate pH 9.6 (1000 ml):
50 mM Na2CO3 pH 9.6 – 6.2 g
Dissolve in 900 ml with distilled water.
Adjust the pH to 9.6 with NaOH.
Make up the volume to 1000 ml.
B. 50 mM Sodium Carbonate pH 10.6 (1000 ml):
50 mM Na2CO3, pH 10.6 – 6.2 gm
Dissolve in 900 ml with distilled water.
Adjust the pH to 10.6 with NaOH.
Make up the volume to 1000 ml.
PBS-BSA (100 ml):
Dissolve 200 mg BSA in 100 ml PBS.
PBS-Tween 20(100 ml):
Add 50 μl of Tween 20 in 100 ml PBS.
Blocking Buffer (1000 ml):
2 % Sucrose – 20 g
0.1% BSA – 1 g
0.9% NaCl – 9 g
Dissolve the contents in 1000 ml distilled water.
Substrate Solution:
Use either horse radish peroxidase HRP, alkaline phosphotase HP system.
Stop Solution:
4M NaOH
Production of Polyclonal Antibody:
A. Immunization of Mice:
1. Inject subcutaneously 15-50 µg antigen (purified protein) in 200 µl Freund’s complete adjuvant (first injection).
2. Two weeks later give a booster dose with 15-50 µg of antigen in 200 µl adjuvant (second injection). (If time permits, boost again after a month).
3. Bleed the mouse 7-10 days later and test serum using the assay which will be used for screening. If titre is not high enough, boost again two weeks after previous boost.
B. Immunization of Rabbits:
Rabbits are immunized essentially.
1. First in Freund’s complete adjuvant 100-200 µg of antigen/1 ml injection, two sites (over each shoulder).
2. Inject second time 1 month later in Freund’s incomplete adjuvant.
3. Bleed two weeks later. Boost again two weeks later (2 + 2 week cycle).
C. Serum Preparation:
1. After collection, the blood should be allowed to clot for 60 minutes at 37°C or overnight at 4°C.
2. Separate the clot from the sides of the tube (ringing) using a pipette. Place it at 4°C overnight.
3. Centrifuge at 10,000 rpm for 10 minutes at 4°C to separate the serum.
4. Serum can be stored at -20°C after adding glycerol to a concentration of 50%.