In this article we will discuss about the requirements and procedure for isolation of frankia.
Frankia is an actinomycete which forms symbiotic association with non-leguminous plants and fix atmospheric nitrogen. There are over 24 genera from eight angiosperm families which have been reported to possess actinorhizal nodules on the root system. Abortive attempts to isolate Frankia in vitro were there from 1910 onwards. Though isolations were made, the cultures did not reproduce nodules. The first successful isolation was by Callaham et al. (1978) where they used cellulose and pectinase to separate active cells from nodules.
Requirements:
1. Fresh nodules from Casuarina.
2. 95% ethanol.
3. 0.1% HgCl2 or 1% NaCl.
4. Sterile distilled water.
5. Slides.
6. Petri dishes.
7. Glass rod.
8. Growth chamber.
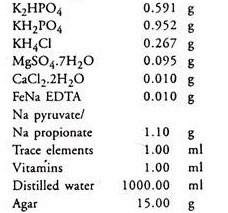
9. Benson’s Ammonium Phosphate medium (BAP) pH 6.7:
10. Trace elements solution:
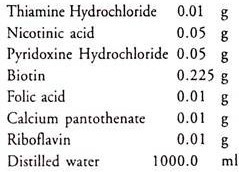
11. Vitamin solution:
Procedure:
1. Excise fresh young nodules from Casuarina roots and clear it from extraneous organic matter soil and dirt by washing it under a stream of running tap water.
2. Fragment the nodules into small lobes keep these in 95% alcohol for 2 minutes and transfer them to 0.1% HgCl2 or 1% Na hypochlorite solution.
3. After 5-8 minutes wash them several times with sterile distilled water.
4. Cut the nodules into small bits by a sterile scalpel and crush them with a sterile glass rod in a drop or two sterile distilled water.
5. Remove the cloud of organism with the help of an inoculating loop and spread on Benson’s ammonium phosphate medium in Petri plates. Also inoculate them in broth with 50 µg/ml of cycloheximide in the medium to prevent fungal growth. The crushed pieces can also be kept in Petri dishes and melted and cooled (45°C) agar medium with cyclo-heximide can be poured on the nodule pieces. This is to provide microaerophilic condition.
6. Seal the Petri dishes with paraffin and incubate at 28°-30°C (room temperature).
7. After about four months small white to pale yellow colonies appear. In broth they grow below the surface.
8. Subculture this on Benson’s slants and broth and use for reinoculation tests (as mentioned for authentication of Rhizobium) and for further studies.