Production of Antibiotics by Fermentation, Bacteria, Fungi, and Penicillin. Also learn about:- 1. Cephalosporins 2. Chloramphenicol 3. Fusidic Acid 4. Griseofulvin 5. Nisin 6. Interferons 7. Tetracyclines 8. Erythromycin 9. Bacitracins.
1. Cephalosporins and Its Fermentative Production:
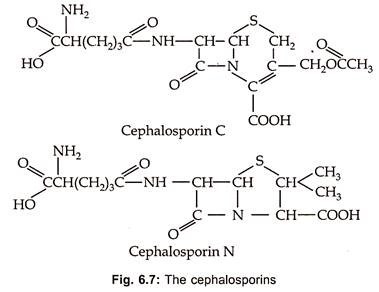
Cephalosporins are β-Iactum antibiotics containing dihydrothiazine ring with D-α-aminoadipic acid as acyl moiety (Fig. 6.7).
Cephalosporin C was discovered in culture filterates of Cephalosporium acremonium in 1953. These are also produced by other fungi such as Emericellopsis and Paecilomyces. In 1971 screening programme designed to discover β-lactum antibiotics, the first cephalomycins (7-methoxy cephalosporins) were produced by species of Streptomyces such as S. lipmanii, S. clavuligerus or Nocardia lactamdurans. These are of most value for their broad spectrum and low human toxicity.
Only semi-synthetic derivatives of cephalosporins and cephamycin are useful therapeutically. These are modified to be used orally with improved β-lactum resistance and are termed as first generation cephalosporins. These are further modified so that they are active even against gram (-) ve bacteria and are termed as second generation cephalosporins. From these a third generation cephalosporins are developed which are with excellent P-lactamase stability and broader action spectrum.
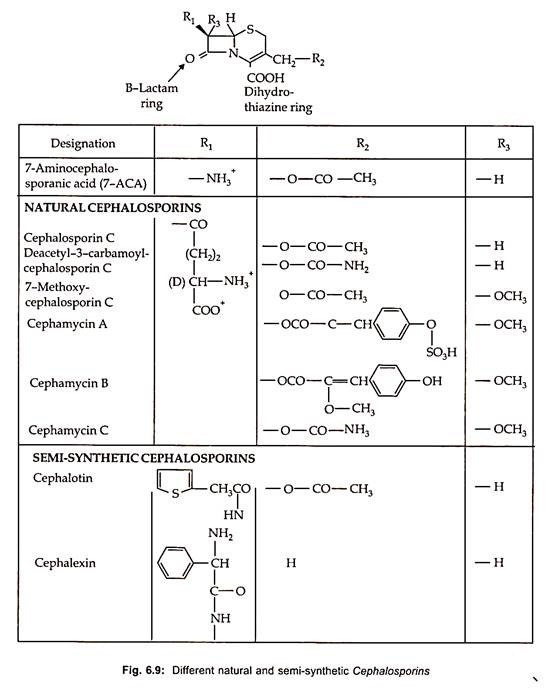
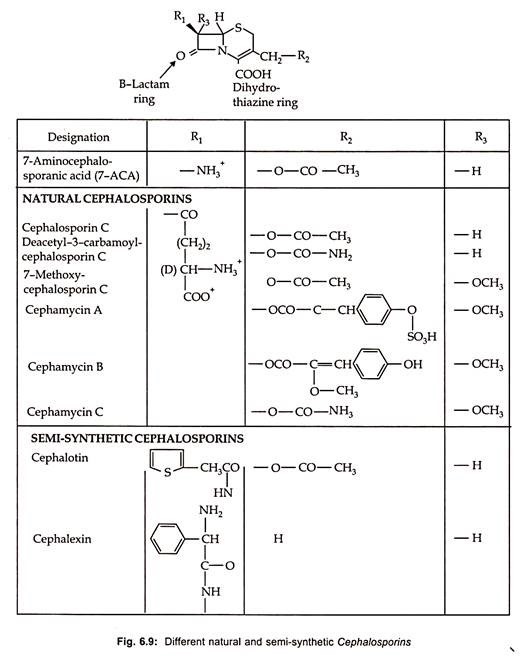
Thirteen therapeutically important semisynthetic cephalosporins are commercially produced by chemical splitting to form 7-aminocephalosporic acid (7-ACA) with subsequent chemical acylation as well as by modification on the C-3 site (Fig. 6.9).
Cephalosporins are as economically significant as penicillins.
Biosynthesis:
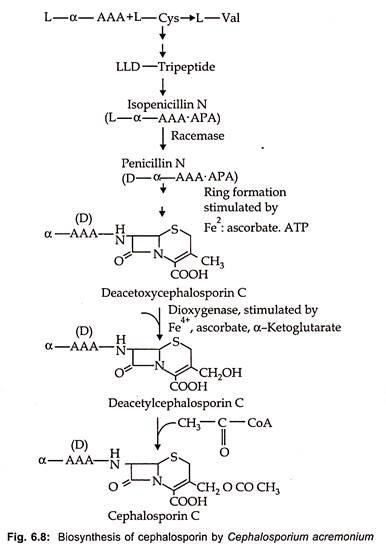
Biosynthesis of cephalosporin proceeds from α (α aminoadipyl) – L-Cystienyl -D-Valine to isopenicillin N as is the case of benzyl penicillin. In the next stage penicillin N is produced by transformation of L-α AAA side chain into the D-form via the action of a very labile racemase. After ring expansion to deacetoxy cephalosporin by expandase reaction, hydroxylation via a dioxygenase to deacetyl cephalosporin C occurs. The acetylation of cephalosporin C by an acetyl CoA dependent transferase is the end point of biosynthetic pathway in fungi (Fig. 6.8).
On the other hand, in Streptomyces further transformation of cephalosporin C or deacetyl cephalosporin C is converted in a two-step reaction with molecular oxygen and S- adenosylmethionine to 7-methoxy-cephalospsorin or cephamycin C. In contrast to penicillin, the D-α AAA moiety cannot be changed with precursor feeding.
The fermentation of cephalosporin is similar to that of penicillin. Complex media with corn steep liquor, meat meal, sucrose, glucose and ammonium acetate are used. Fermentations are carried out as fed batch processes with semi-continuous addition of nutrients, at pH 6.0 – 7.0 at a temperature between 24-28°C. High aeration is necessary in main growth phase and O2 absorption decreases sharply during production phase.
Chemical synthesis of cephalosporins by ring expansion of penicillin has been developed which is more economical. By using pencillin V, oraspor, an orally active cephalosporin is produced. Similarly, some of the semi-synthetic cephalosporins along with their structure and characteristics are illustrated in Fig. 6.9.
2. Chloramphenicol and Its Fermentative Production:
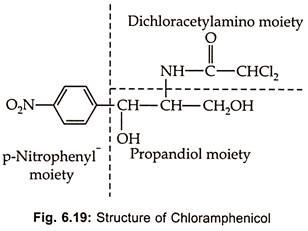
Chloramphenicol, an aromatic antibiotic Fig (6.19), is produced by Streptomycetes venezuelae S. Phaeochromo-genes chloromyceticus var S. omiyarnsis and other streptomycetes.
It is a broad spectrum antibiotic acting on both gram (+) ve and gram (-) ve, actinomycetes, rickettsiae and Chlamydias. Because of its toxic effect on bone marrow chloramphenicol has not been used widely. However, it is an indispensable in the treatment of persistent typhoid due to intracellular Salmonella typhi which does not respond to penicillin and other antibiotics. It’s toxicity can be reduced if therapy is conducted carefully.
Chloramphenicol binds specifically to the 50s subunit of 70s ribosomes and blocks the peptidyl transferase reaction causing premature chain break. It is used in molecular biology as a research to study translation in prokaryotes without affecting nucleic acid synthesis.
Chloramphenicol is synthesized via aromatic pathway from chorismic acid. It has been successfully synthesized chemically since 1950 and proved to be more economical.
Fermentation:
The fermentation is carried out in 30 liters fermenter containing 18 liter medium consisting of glycerol (1%), yeast extract or tryptone, sodium chloride (0.5%) and pH is adjusted to 7.5. The fermentation is carried out at 25°C for 3-4 d. The highest yield obtained was 200-300 mg l-1.
Chloramphenicol is extracted from the clarified broth. The filterate is extracted either with ethyl or diluted with kerosene and then washed with dilute acetic acid, sodium carbonate and water. The lipids are removed by petroleum ether. The crude product is decolorized by passing the organic solution through a column of charcoal or alumina. The purified product is recrystallized from ethylene or ether and petroleum ether mixture.
3. Fusidic Acid and Its Fermentative Production:
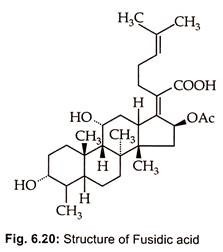
Fusidic acid, a steroid antibiotic (Fig. 6.20), is produced by Fusidium coccineum.
It is therapeutically important in the treatment of staphylococcal infections, specifically wound infections and those of skin infections.
Fusidic acid, is reported for the first time by Godfredsen et ah, (1962) to be produced by Fusidium coccidium by species of Cephalosporium and Mucor ramannius.
Fusidic acid has been used extensively in the treatment of infections caused by penicillin resistant staphylococci.
It inhibits t-peptidyl transferase in both pro – and eukaryotes and affects EFG:GDP ribosome complex by forming stable complex. It is also an uncoupler of oxidative phosphorylation (inhibits ATP synthesis). Fusidic acid is most useful in the treatment of β-lactamase resistant staphylococci.
4. Griseofulvin and Its Fermentative Production:
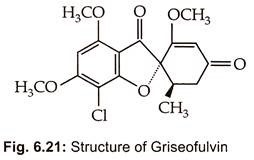
Griseofulvin GN was reported by Oxford et al. (1939) from cultures of Penicillium griseofulvum. It is now known to be produced by several species of Penicillium. Commercial product of griseofulvin by high yielding strain of Penicillium patulum. Its chemical structure (Fig. 6.21) was elucidated by Grove et al. (1952) and is 25 trans -7 chloro -2,4,6 trimethoxy 6-methylspiro – (benzofuran -2 (3H)-1-2-cyclohexane-3.4′- dione).
It inhibits net proteins, carbohydrates, lipids and RNA without affecting DNA synthesis. These levels cause regular curling of hyphae, forms of binucleate and multinucleate cells. GN caused mutation in Microsporus gypseum. In general GN inhibits mitosis in fungi by affecting microtubules of nuclear spindle.
Oral administration of GN controls infection of skin, hair and nails caused by Microsporum, Trichophyton and Epidermophyton. Griseofulvin is known to bind strongly to keratin of the skin. Although griseofulvin is useful in the treatment of skin infections it cannot be used in systemic mycosis.
Fermentation:
P. griseofulvum is grown in 3 different media:
(i) Sporulation medium (whey powder lactose (30.0g), NaNO3 (3.0g), KH2PO4 (1.0g), MgSO4.7H2O (0.5g) and FeSO4.7H2O (0.02g),
(ii) Germination medium (protopeptone (20g), malted cereal extract (10.0g), glucose (40.0g), starch (20.0g), NaNO3 (3.0g), KH2PO4 (1.0g), MgSO4 (0.5) and FeSO4 (0.02) and
(iii) Seed stage medium (corn-steep liquor (3.0g) brown sugar (30g), chalk (10.0g), corn oil (10.0g) and Hodag M.F.(0.3g).
For production of griseofulvin medium composition is corn steep liquor (0.17g) CaCO3 (0.4) KH2PO4 (0.4) and KCl (0.15). For good yield production of griseofulvin 12% carbon source, 0.4-0.5% phosphate and chlorine is required during fermentation. The pH is maintained at 6.8, and 3.2 incubation temperature was 25°C and it is an aerobic fermentation. Yield is 6-8g l-1 after 10 days after addition of methyl donors such as chlorine salts, methyl xanthate and folic acid to the medium.
Griseofulvin is extracted from the mycelium with organic solvent and butyl acetate and evaporated to dryness. The crude griseofulvin is washed with chloroform and recrystallized from aqueous methanol. Methylene chloride gives yield upto 95% and purity upto 88%.
5. Nisin and Its Fermentative Production:
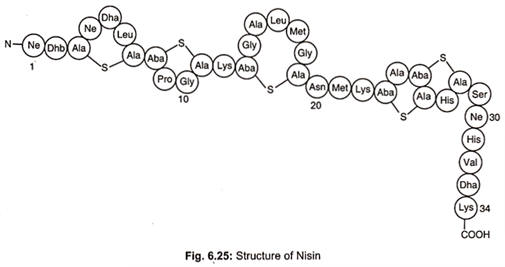
Nisin is a primary metabolite. It is a member of well characterized peptides termed lantibiotics which contain unusual dehydro residues and thioether amino acids that introduce the characteristic lanthionine rings into the peptide chain (Fig. 6.25).
Nisin, a bacteriocin, is produced by Lactococccus lactis. It is amphilic polypeptide, pentacylic peptide of 34 amino acids with a molecular weight of 3500 daltons. It contains unusual amino acids including one lanthionine, four β- methyl lanthionine, one dehydrobutyrine (Dhb) and two dehydroalanine (Dha) residues. Dha and Dhb arise from dehydration of L-serine and L-threonine respectively.
Condensation of Dha and Dhb with L-cystine generates thioether bonds to form the amino acids lanthionine and B-methyl – lanthionine, respectively. These changes result from extensive post- translational modifications to the ribosomally synthesized 57 amino acids, residual precursor peptide, prenisin. The active nisin molecule is finally formed and released from the cell after cleavage by protease to 34 amino acids peptide.
On the post-translational modification nisins can be classifed into two types:
Type I:
Lantobiotic bacteriocines undergo extensive post translation modification e.g. Nisin.
Type II:
Non-Lanthionine bacteriocines undergo minimal post translational modifications e.g. lactococcine and pediocine.
The gene for nisin biosynthesis along with immunity to nisin, nisin transport, serine properties such as facility to ferment sucrose are organized as an operon that is encoded on a chromosomally located 70kb conjugative transposon. Nisin acts on membrane resulting in the leakage of low molecular weight materials i.e. ions, amino acids and ATP.
Nisin molecules enter into a susceptible organism and congregate in groups and then form pores. This also depletes the electric charge stored across the membrane and the bacterium begins metabolizing ATP to produce new protons in a futile effort to recharge the membrane eventually depleting its ATP molecules and unable to maintain its other activities and quickly dies.
It is very active against Gram (+) ve bacteria such as Bacillus, Clostridium, Desulfomaculum, Enterococcus, Lactobacillus, Leuconostoc, Listeria, Micrococcus, Pediococcus, Staphylococcus and Sporolach. Individually, it is inactive against G (-) ve bacteria, yeasts and filamentous fungi. It is very active against Bacillus sporothermodurans which is problematic in ultra-high temperature treated products.
It is extensively used in controlling bacterial spoilage of both heat processed and low acid pH foods, which include dairy products, specially processed cheese, cheese spreads, egg products, dressings, along with several canned foods, nisin is particularly useful in controlling our growth of endospores of Bacillus and Clostridum. It is also used in heat sanitizers for the prevention of bovine mastitis in dairy cattle.
It has potential to control oral bacteria responsible for mouth odour, plaques, acid gingivitis. It can be used as skin care protective agent in the control of skin infection of Staphylococcus aureus and S.pyogenes. It has a possible role in the control of Helicobacter pylori which infects the stomach mucosa and is associated with 90% of peptic ulcers.
Nisin Production:
Nisin is manufactured by controlled fermentation of Lactobacillus lactis in a milk based medium at pH 2.0. Above pH 3.0 nisin is absorbed by the producer cells, but it is completely destroyed at pH 3.0 or below. Consequently at the pH of the fermentation all nisin is released into the medium from which it can be extracted at the end of fermentation. Solvent extraction methods have been used and one step immuno-affinity chromatography method is highly efficient.
However, in the industrial scale process, it is concentrated and separated by a simple low-cost foaming process. This involves merely bubbling nitrogen or air through a column of completed fermentation medium. As nisin is a surface active agent, it accumulates in the foam at the air aqueous interface. The foam is collected, broken mechanically and the nisin is recovered. It is then spray dried before being milled into fine particle and finally standardized by the addition of sodium chloride.
Bacitracins are most useful in the preservation of-
1. Milk and dairy products;
2. Meat and poultry products;
3. Fish and sea foods.
1. Milk and Dairy Products:
(i) Reduces microbial growth in milk,
(ii) Inactivates mesophytic bacteria,
(iii) In fermented dairy product it inhibits gas forming Clostridium tyrobutryicum and pathogenic bacteria such as listeria monocytogenes, Bacillus cereus and staphyloeccus aureus in cheese. It also controls over production of acid in yogurt and accelerates cheese ripening by releasing increased bacterial intracellular enzymes. It also inhibits endospore formation in processed dairy products.
2. Meat and Poultry Products:
In contamination of raw meat and extends self-life vacuum packaged raw meat and inhibits the growth of listeria monocytogenes. It inhibits the spoilage lactic acid bacteria and other bacteria including pathogenic bacteria in cooked meat products. Similarly it is useful in increasing shelf life of egg products. In fermented meats it is useful in the control of spoilage lactic acid bacteria and pathogenic bacteria like salmonella, listeria and staphylococcus and promotes the growth of starter cultures.
3. Fish and Sea Products:
It is in combination of other bacitracins it is extensively used in the control of fish and other sea foods.
6. Interferons and Its Fermentative Production:
Interferons are antiviral, non-antibody proteins synthesized and secreted by vertebrate cells in response to virus infections and other stimuli. Cells exposed to interferons respond by acquiring resistance to subsequent virus infection. These are first described as biologically important regulating proteins called cytokines.
Interferons also able to inhibit the proliferation of cancer cells because of these combined properties of inducing antiviral resistance and regulating cell growth, interferons are used as therapeutic agents for the treatment of both viral infections and cancer. Interferons are able to modulate the immune response and this has led to their clinical use in diseases underlying immunological etiologies including multiple sclerosis.
Interferons were discovered in 1957 as antiviral agents synthesized in influenza virus infected chick embryo cells. Interferons are produced by cells in response to viral infection and confer an antiviral state on cells exposed to them. In addition to avian, interferons are also present in fish, reptiles and mammals.
Interferons are of two types:
1. Type I:
Interferons is predominantly 1FN α and β but it also includes two other sub family members IFN ω’ and IFN γ.
2. Type II:
Comprises only 1FN- γ or earlier known as immune or type 2 interferon which is produced by lymphoid cells in response to mutagens and by sensitized lymphocytes when stimulated with specific antigen.
Interferons are primarily produced by leucocytes and they consist of a single polypeptide chain of 165-166 amino acid residues. Some are glycosylated with varying amount of carbohydrate moieties and their molecular mass is in the range of 16000-26000Da. The carbohydrate portion does not appear to confer any functionality on IFN α and may be removed without affecting their activity.
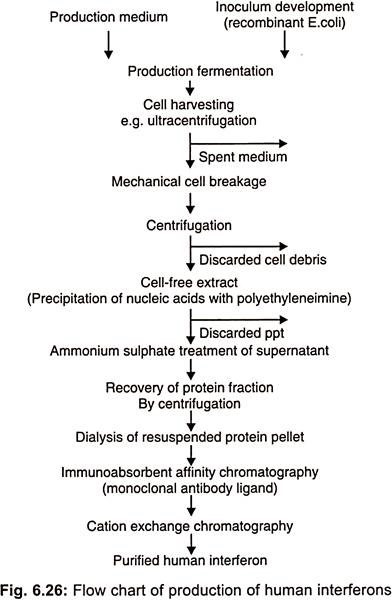
This property allows recombinant 1 FN – α to be produced in prokaryotic systems such as E.coli which are not capable of the post transcriptional modifications necessary to form glycosylated polypeptides (Fig. 6.26).
Recombinant 1FN- α is now manufactured by a number of companies for example Hoffmann-La Roche produce Roferon and Schering plough make intron which have combined sales worth in excess of US $ 750 million.
IFN β is naturally synthesized by mammalian fibroblast cells and can be produced recombinantly from E.coli. These products such as Betaseon and Rerif are used in the treatment of relapsing multiple sclerosis. IFN- γ sometimes referred to as immune interferon is produced naturally by activated lymphocytes. It is most important interferon involved in the immune system where it activates helper cell (TH1), natural killer cells (NK), cytotoxic T-lymphocytes (CTL) and macrophages and also exhibits antiviral and oncostatic properties.
Preparations of recombinant IFN γ from E.coli e.g. Genenechs Actimmune, are used in the treatment of chronic myeloid leukemia and renal cancer.
Applications of Interferons:
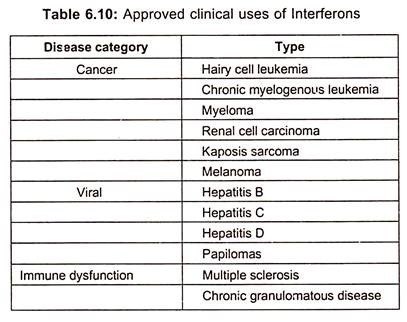
Though interferons hold a promise of treatment of viral diseases, it was not realized until the production and testing of recombinant interferons which are now approved for the treatment of hepatitis B and C. Interferon α is the only effective treatment for patients with chronic hepatitis C. However, it has approval of interferons (type I) for a range of malignant, viral and autoimmune diseases (table 6.10).
In addition to hairy cell leukemia, both natural and recombinant interferons are used for treating chronic myelogenous leukemia, melanoma, renal cell carcinoma, lymphoma and karposis sarcoma.
Interferons are either used alone or as adjunct therapy and new methods for stabilizing interferon, increasing bioavailability are impacting on the clinical utility. In addition to viral hepatitis, interferons are also used for treatment of herpetic keratitis and laryngeal and genital papillomas.
Although use of interferon in multiple sclerosis was quickly terminated because of adverse outcomes, it is approved for treating chronic granulomatous diseases. Recombinant interferon β produced either as an unglycosylated form in E.coli or as glycosylated protein in mammalian cell cultures is used extensively in the treatment of relapsing/remitting multiple sclerosis.
Allergic Responses:
Interferons, however, cause some adverse side effects such as chills/ rigors, fever, myalgia’s and mild neutropenia with initial injections, while fatigue anorexia, mild neutropenia, elevation of transaminase, weight loss and depression due to chronic administration.
7. Tetracyclines and Its Fermentative Production:
Chlortetracycline was discovered in 1945 produced by Streptomyces aureofaciens. By its dehalogenation, tetracycline was prepared which was subsequently produced by S. viridifaciens. Presently about 20 streptomyces species are known to produce mixture of different tetracyclines.
Biosynthesis:
Tetracyclines such as chloro and oxytetracyclines are produced by species of Streptomyces and tetracycline usually being only a minor component. However, S. aureofaciens mutants with a block in the chlorination reaction excrete tetracycline as the major product.
The biosynthesis can be subdivided into three sections:
1. Glucose is converted into acetyl-Co A
2. Malonyl CoA is produced in the transformation of acetyl – CoA by means of acetyl CoA – carboxylase. After transamination to malonyl CoA, which is bound to an enzyme complex (anthracene synthase), this compound condenses with 8 mol. of malonyl CoA.
The polyketide assembly formed from this condensation has not isolated. An alternate pathway to malonyl CoA is via the production of oxaloacetate catalyzed by PEP carboxylase oxaloacetate being converted to malonyl CoA by oxidative decarboxylation. Cyclization into the tricylic intermediate also takes place on the enzyme anthracene synthase.
3. The step by step transformation of the assumed tricyclic intermediate, which has not yet isolated, into variety of known tetracyclines.
The scheme of biosynthesis of chlortetracycline, still mainly hypothetical, has been deduced from studies of mutants blocked in tetracycline biosynthesis and from co-synthesis studies with S. aureofaceins. Out of 72 intermediate products, only 27 substances could be characterized and the chlorination reaction is itself not yet understood.
A close correlation could be observed between carbohydrate metabolism and tetracycline production. High yielding strains have lower glycolysis rate than the low yielding strains. The addition of 0.5-3.0 µg ml-1 benzylthiocynate covers 50% increase in chlortetracycline production and pentose phosphate cycle increases simultaneously. Pentose-phosphate regulates tetracycline biosynthesis.
The addition of phosphate to producing culture decreases the rate of tetracycline formation by inhibition of ATC oxygenase. The rate of tetracycline synthesis is directly proportional to the activity of this enzyme, which is only detectable in the culture after phosphate has depleted completely.
Strain Development:
Over 300 genes are reported to be involved in the biosynthesis of chloro- tetracyclines. UV radiation alone or in combination with other mutagens has brought about the greatest number of high yielding strains of S.aureofaciens and S.ramosus. The increase in chlortetracycline production with each step of mutagens treatment in the course of strain development is depicted in Fig. 6.15.
Strain with increased tetracycline production have been isolated by selecting revertants of mutants blocked in antibiotic synthesis and from prototrophic revertants of auxotrophs. They may also be isolated from mutants showing increased resistance to their own product. The reported highest yield for tetracycline is 25 glt-1. Hybridization alone has not yielded an improvement in tetracycline production, but in combination with mutagen treatment has resulted an increase in its production. Protoplast fusion studies have also resulted in the increased production.
Genetic Regulation of Tetracycline Biosynthesis:
1. Three hundred genes are reported to be involved in biosynthesis of CTC.
2. First step of oxytetracycline biosynthesis is located at rib b and cyc d.
3. Genes for steps from a hydrotetracycline to oxytetracycline are located in second cluster.
4. Pro A and add A clustering of genes facilitates genetic engineering research.
5. Recombinant – DNA technology has been developed for oxytetracycline producing strain of S.ramosus.
Fermentation Production:
1. By a chemical process using chlortetracycline.
2. By fermentation in chloride-free culture medium, which can be made by pretreatment with ion exchangers.
3. By adding chlorination inhibitors to medium such as mercaptobenzothiazole, 2- thiouracil or thiourea. Bromide inhibits chlorination in some cultures but causes formation of 7-bromotetracycline in other strains.
4. With mutants which are blocked in the chlorination reactions.
Production of tetracyclines is carried out in stirred fermenters with volumes upto 1.5 lakhs liters. Typical process of production of chlortetracycline is depicted in Fig. 6.16.
If glucose is used, continuous feedings is necessary, starch can be used as an additional carbon source. Tetracyclines are produced in phosphate limitation. This fermentation is aerobic and production increased with the increase of oxygen concentration.
8. Erythromycin and Its Fermentative Production:
Erythromycin is one of the macrolides which are lipophilic with 14 membered macrocyclic lactone ring. Besides, erythromycin, josamycin, leucomycins, decamycin, oleandomycin, spiramycins and tylosine are other antibiotics belong to this group. Macroslides are hydrophobic and usually basic compounds.
Structure:
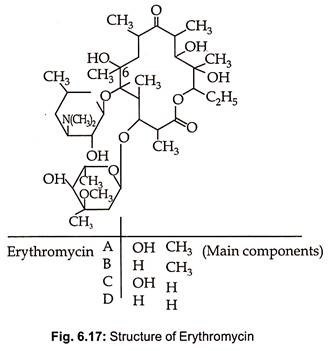
Erythromycin is produced by Streptomyces erythreus, St. griseoplanus and species of Micromonospora. Erythromycin B, C and D are produced as minor products during fermentative production of erythromycin A. They consist of 12, 14, 16 and 17 numbered lactone rings with 1-3 sugar glycosidically linked with the glycone (lactone ring) and with each other. The sugars are amino sugars and/ or 6 deoxyhexoses. The structure of erythromycin, a 14 membered macrotide, is given in Fig. 6.17.
Erythromycin is very effective against Gram (+) ve bacteria particularly Staphylococci and mycoplasmas, were frequently used against penicillin resistant organisms. Because of the development of the newer β-lactum antibiotics, the macrolides are no longer of great medical significance. The aglycone and sugar moieties are both necessary for antibacterial activity.
Therapy with erythromycin results in a rapid development of resistance and there is also cross- resistance between the individual antibiotics. However, because of low toxicity, efforts are being made to isolate new compounds of this class and also to modify known macrolides through biotransformation.
Erythromycin is an inhibitor of protein synthesis, binding to the 50s subunit of bacterial ribosomes. Erythromycin is known to inhibit the elongation factor G-dependent release of deacylated tRNA from the p-site of the ribosome.
Biosynthesis:
The aglycone moiety of erythromycin is built either of propionate subunits or of a combination of acetate and propionate subunits. Sometimes butryate or related compounds participate in aglycon moiety formation. Details of biosynthesis of erythromycin are available from labelling studies with S. erythreus, Erythronolide is produced from propionyl – CoA onto which 6 molecules of 2 methylmalonyl -CoA condense.
This polyketide synthesis takes place on a multi enzyme complex called lactone synthetase which is very similar to the fatty acid synthetase of S. erythreus. After cyclization to the aglycon, the first compound, 6-deoxyerythronolide B can be isolated. 6-deoxyerythronolide B is further glycosylated to produce erythromycin C and erythromycin B (Fig. 6.18).
Fermentation:
Erythromycin is produced in an aerobic submerged fermentation using St. erythreus. Yield in large scale industrial processes are around 20 gl-1. The production medium contains glucose 50 g, soymeal 30 g, (NH4)2 SO4 3 g, NaCl 5 g, CaCO3 6 g, tap water 1 liter and pH 7.0. Complex culture medium ingredients may also be used in the medium. Fermentation temperature is 33°C and the incubation period ranges from 3 to 7 d. The inoculum from the culture grown on tryptone plates with suspension of at least 108 viable cells ml-1.
Erythromycin production starts when growth reaches the stationary phase, consumption capacity of inorganic phosphate, ammonium nitrogen, metal ions as well as nitrogen source increases and at high concentration of dissolved oxygen. The addition of n-propanol as precursor increases erythromycin titer. The oxygen requirement during 0-12 hrs is 0.4 v/v per min and increases to 0.9 v/v per min from 12 hrs to till harvest. Carbon dioxide is added at 0.1 vol/vol/min or 11% of the inlet air which increases the erythromycin yield to 40% of the control.
Erythromycin is produced extracellularly and can be separated by filter press, centrifuge or drum filter with a filter aid. The acidic condition helps to separate mycelium from the broth. Erythromycin is extracted using methyl isobutylketone or ethyl acetate. It is then transferred to acidic water. pH is adjusted with HCl, phosphoric acid, acetic acid or citric acid. The purification and concentration is carried with ion exchange resin i.e amberlite. Further purification step of antibiotic is adsorbed on resin and is eluted by a mixture of organic solvents and water at pH 3.0-8.0.
Production of erythromycin is reported to involve three genes each of which is predicted to code for a protein of more than three lakhs daltons and six molecules each of which in turn contains acyl carrier protein, acyl transferase, keto acyl ACP-synthetase and enoyl – ACP synthetase as well as other domains needed for biosynthetic process.
9. Bacitracins and Its Fermentative Production:
Bacitracins, polypeptide antibiotics, are produced by a group of bacteria which are active only on closely related organisms. Gratia (1925) was the man who discovered bacitracin produced by E.coli which was effective against another strain of E.coli. The same was renamed as colchicine by Gratia and Fedriq (1946). In addition to its use as a topical antibiotic, it is also used as a growth promoter in animal feeds.
As an animal feed supplement, it can be used as the zinc or manganese salt, as the lignin bacitracin complex or as bacitracin methylene di-salicylate. They exhibit activity primarily against closely related species. They are attached to specific cell receptors and their genetic determents were plasmid encoded.
The definition includes that the synthesis of bacitracins was lethal to producer. Bacitracins are given names according to the genus or species of producing organism eg. Pediococcus- pedicines and Clostridium botulinum- bollicine.
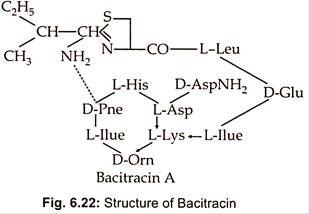
Different strains of Bacillus licheniformis produce a mixture of bacitracins such as bacitracin A.A,B,C,D,E,F,F1,F2,F3 and G. Bacitracin is the main component (about 70%) and has the greatest biological activity. It is a dodecapeptide consisting of L and 4D -amino acids and contains both a cyclic hexapeptide and a thiazoline ring structure (Fig. 6.22).
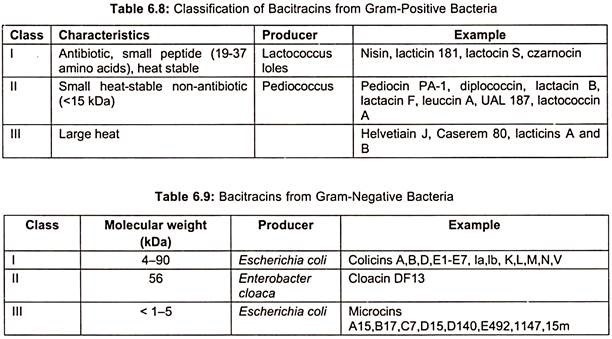
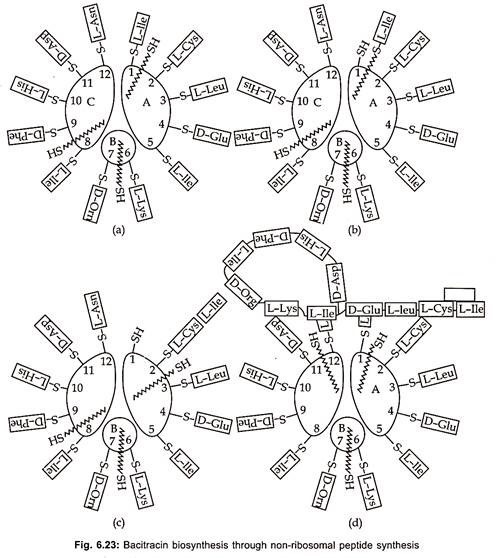
Bacitracins are also produced by both Gram (+) ve bacteria (table 6.8) and Gram (-) ve bacteria (table 6.9). Total production of bacitracins worldwide was estimated to be 500 tons. Peptide formation can occur by either ribosomal or non-ribosomal mechanisms. Most peptide antibiotics does not involve this protein-synthesizing machinery, but takes place on a multi-enzyme complex. This non-ribosomal peptide synthesis process which has also been called the thiotemplate mechanism is described here for bacitracin (Fig. 6.23).
Bacitracin synthesis consists of subunits A, B and C with molecular weights of 20,000, 2,10,000 and 3,80,000 daltons respectively. The first step is the activation of amino acids in the presence of ATP and Mg to aminoacyl adenylate and takes place at a specific activation site for each amino acid on the enzyme complex. The activated amino acids are then bound to specific thiol groups in thioester linkages on the enzymes.
Various strains of Bacillus licheniformis produces mixture of bacitracin A, B, C, D, E, F and G. Bacitracin A which is dodecapeptide, has greatest biological activity. It inhibits the formation of the bacterial cell wall at the level of peptidoglycan synthesis. It prevents dephosphorylation of C55 – isoprenyl pyrophosphate to C55 isoprenyl- phosphate. The amino acid sequences of the subsequent peptide is determined by the arrangement of thiol groups in thioester linkages on the enzyme.
The racemization to D- enantiomers probably take place at this level. The transfer of the growing peptide chain (growth of the N-terminal toward the C-terminal end) to the next amino acid and to the next enzyme sub- unit is catalyzed by 4′ phosphopantheine, a cofactor, of each subunit. The cyclization of the peptide chain takes place in the multi-enzyme complex, but the formation of thiazoline ring is not yet fully understood.
Fermentation Production:
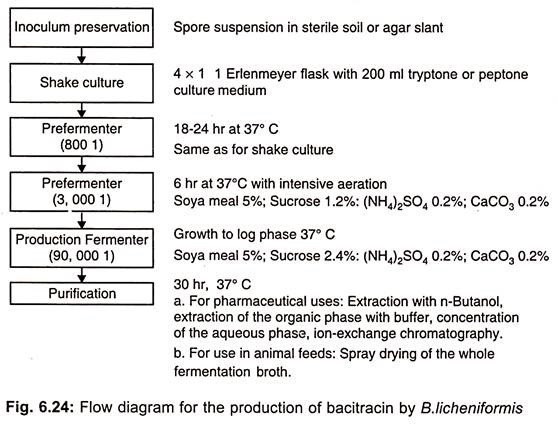
Bacitracin is now being produced by aerobic submerged culture. Production of bacitracin is précised in (Fig. 6.24).
The yields of this process were increased from about 13 mg liter-1 to about 9g liter-1 through strain development and medium optimization. A continuous fermentation process has also been developed for bacitracin production using immobilized cells.
Bacitracins are extensively used as food and feed supplement.
Bacitracins intended for use as food preservatives should fulfil following conditions:
1. Proven safe for human consumption.
2. Economically acceptable cost.
3. Proven effective at relatively low concentrations.
4. No detrimental effect on organoleptic characteristics of the food.
5. Stable during storage and effective for the self-life of the food.
6. No medical use.