In this article we will discuss about:- 1. Introduction to Penicillin 2. Biosynthesis of Penicillin 3. Structure 4. Fermentation Process 5. Uses.
Introduction to Penicillin:
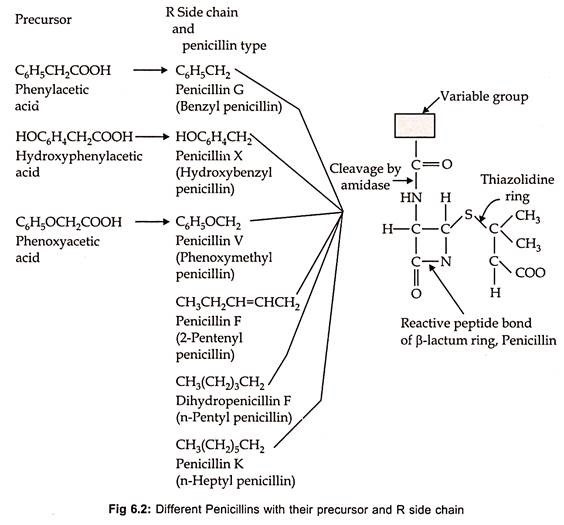
Chemically the natural penicillin is 6-amino penicillanic acid (6 – APA), which consists of thiazolidine ring with a condensed β-lactum ring. The various penicillins differ primarily in the nature of R-side chain which are attached by an amido linkage to the chemical nucleus of the molecule. Fleming’s original Penicillium notatum strain, when grown on his medium produced penicillin-F, which is known as 2-pentinyl penicillin.
Subsequently P. chrysogenum proved to be better fungus and more suitable for submerged fermentation. The basic structure of penicillin and different types of natural penicillin’s differing in the composition of side chain are shown in Fig. 6.2.
If penicillin fermentation is carried out without the addition of side chain precursor, the natural penicillins are formed from which only benzyl penicillin can be isolated. However, the desired penicillin can be obtained by adding suitable side chain precursor into the medium. Such penicillins are called as semi-synthetic penicillins.
Penicillin-G and Penicillin-V are generally produced commercially. When compared to natural penicillins, semisynthetic penicillins have improved characters viz, acid stability, resistance to plasmid or chromosomally coded β-lactamases, expanded antimicrobial effectiveness and are therefore, extensively used in therapy.
Biosynthesis of Penicillin:
The β-lactum thiazolidine ring of penicillin is formed by the condensation of L-cystine and L-valine. The biosynthesis occurs in a non-ribosomal process by means of dipeptide composed of (α – α – AAA) and α-cystine or a breakdown product of cystothiamine. Subsequently L-valine is connected via epimerization reaction resulting in the formation of tripeptide. The first product of cyclization of the tripeptide which can be isolated is isopenicillin N but the biochemical reactions leading to this intermediate is not understood. Benzyl penicillin is produced in exchange of α -α-AAA with activated phenylacetic acid (Fig. 6.3).
About 38% of the penicillins produced commercially are used as human medicine, 12% in veterinary medicine and 43% as starting materials for the production of semi-synthetic penicillins.
Structure of Penicillin:
Some of such semisynthetic penicillins along with structure and biological activity are presented in table 6.4 and Fig. 6.4.
Hetacillin, bacampicillin, epicillin, pivampicillin, and talampicillin are converted to ampicillin in the body. These penicillins exhibit various improvements including resistance to stomach acids to allow oral administration, to pencillinase and an extended range of activity against gram (+) positive bacteria.
It has been reported that most of the high yielding strains of P. chrysogenum are genetically unstable. Genetic unstability increases with the increase in the yield. However, it can be controlled to some extent by following suitable preservation methods. The following preservation methods are generally adopted for storing high yielding strains of P. chrysogenum.
1. A spore suspension is stored in a frozen state under liquid nitrogen.
2. A spore suspension can be lyophilized in an appropriate medium.
3. A spore suspension is mixed with a sterile finely divided inert material like soil or sand and desiccated.
Fermentation Process of Penicillin:
Penicillin fermentation is an aerobic process with a volumetric oxygen absorption rate of 0.4 -0.8mm min-1. The required aeration rate varies according to the strain, the type of fermenter used and on the impellor system. However, the aeration rate varies between 0.5 and 1.0 vvm. It is produced by fed batch submerged fermentation in a stirred tank fermenter.
This process can be described under following headings:
1. Strain development,
2. Inoculum production,
3. Inoculation,
4. Extraction and purification
1. Strain Development:
The variety of molds which yield greater amount of penicillin is called as high yielding strain. They are generally developed from the wild P. chrysogenum by a process called sequential genetic selection. This process consists of stepwise development of improved mutant by treating the wild strain of P. chrysogenum with a series of mutagenic agents or exposing to ultraviolet radiation either individually or in combination, such as X-rays and chemical mutagens, is called as strain improvement.
Strain development is a laborious and time-consuming process. The selected mutant possesses greater capacity for antibiotic production than the wild type.
The expanded role for penicillins came from the discovery that different biosynthetic penicillins can be formed by the addition of side chain precursors to the fermentation medium and that natural penicillins can be modified chemically to produce penicillins with improved characteristics. Most penicillins are now semisynthetic produced by chemical modification of natural penicillin obtained by fermentation using strains of P chrysogenum.
Modification is achieved by removing their natural acyl group, leaving 6 APA to which other acyl groups can be added to confer new properties. This is achieved by passage through a column of immobilized penicillin acylase usually obtained from E.coli at neutral pH. Penicillin G for example converted to 6-APA and phenylacetic acid. The 6-APA is then ethically acylated with an appropriate side chain to produce a semi-synthetic penicillin.
2. Inoculum Production:
The microorganism which is used in a fermentation process is called as the inoculum. A high yielding strain of P. chrysogenum is generally employed as inoculum.
A strain of the fungus is sub-cultured from stock culture for inoculum development. Spores from primary source are suspended in water or in a dilute solution of a nontoxic wetting agent such as 1:10000 sodium lauryl sulfate. The spores are then added to flasks or bottles of wheat bran plus nutrient solution and these are incubated for five to seven days at 24°C so as to provide heavy sporulation. The entire process is repeated several times in order to have more sporulation.
The resulting spores are used directly to inoculate inoculum tanks or stirred fermenters. The incubation temperature is maintained at 24-27°C for 2 days with agitation and aeration in order to facilitate heavy mycelial growth, which may be added to a second or even a third stage fermentation.
The resulting inoculum which is employed in a production tank is tested both by microscopic examination and by sub-culturing method. Many sporulation media have been designed to obtain large number of spores. The one developed by Moyer and Coghill (1946) is most extensively used and given below (table 6.5).
3. Inoculation:
Introduction of pure inoculum into the production tanks or fermenters is called as inoculation.
This is done by any one of the following three methods:
1. Dry Spores may be used as Inoculum:
Since the spores of P. chrysogenum are hydrophobic, either spores are blown deep into the medium or a wetting agent such as sodium lauryl sulphate is used.
2. Suspension of Ungerminated Spores:
This suspension is made by using 1:10000 sodium lauryl sulfate solution. This suspension is fed to the fermenter by suitable techniques like spray guns or pipettes. This is followed by agitation and aeration of the fermentation medium in order to achieve equal and uniform distribution of the spores in the entire medium.
3. Feeding the fermentation tanks with pre-germinated spores or mycelial pellets which are prepared by the germination of spores. Pellets are generally fed to the fermentation medium after two or three days of spore inoculation.
Fermenters with a capacity of 40,000 to 2 lakhs liters are generally employed for the production of penicillin. Due to difficulties with the oxygen supply larger tanks are not employed. Some manufacturer’s use of Waldh of fermenters or air lift fermenters, but this is only possible in mutants which generate low viscosity. Depending upon the production strain, the operational temperature is maintained between 25°-27°C. A typical flow chart for penicillin production is given in Fig. 6.5.
(ii) Medium:
The medium employed for penicillin production should be suitable to achieve:
1. An abundant growth of the mycelium.
2. Maximum accumulation of the antibiotic.
3. Easy and inexpensive extraction and purification of the antibiotic.
Carbon source is generally supplied in the form of lactose. Glucose, sucrose, glycerol and sorbitol can also be employed as carbon source. Nitrogen source is generally supplied in the form of ammonium sulphate or ammonium acetate or ammonium nitrate. Abundant formation of mycelium and spores takes place when a medium contains corn-steep liquor because it contains important amino acids required for mycelial growth.
Potassium, phosphorus, magnesium, sulphur, zinc and copper are supplied in the form of salts. Potassium and phosphorus are supplied in the form of potassium dihydrogen phosphate, magnesium, iron and copper are supplied in the form of sulphates. All these elements may be present in corn steep liquor.
Penicillin-F and penicillin-K are the naturally produced penicillins synthesized by P. notatum and P. chrysogenum, respectively, in the absence of precursor. But, if phenylacetic acid is supplied in the medium P. chrysogenum produces penicillin-G instead of penicillin-K. Similarly, desired synthetic penicillins can be obtained by adding the medium with suitable precursor.
A medium designed by Jackson (1958) which has the following composition, is generally used in fermentative production of penicillin (table 6.6).
Penicillin yields with time are linear from approximately 48 to 96 hours. The final penicillin yield is in the range of 3 to 5% which largely depends upon the amount of carbohydrate consumed during fermentation process, which is approximately equal to 1500 international units per milliliter. Sylvester and Coghill (1954) have estimated that to produce 1000 gallons of fermented culture, which is capable of yielding 2.2-2.7 kg of penicillin by the submerged culture method requires approximately 227 kg of nutrients, 3400 kg of steam, 45460 lt of water, 1000 kWh of electricity and 7075 m3 of air.
Penicillin easily get carboxylated to form penicillianic acid which is biologically inactive by the action of enzyme penicillinase. The enzyme penicillinase is widely distributed among different microorganisms. These organisms may enter into the fermenter at any stage and may convert penicillin into penicillianic acid (Fig. 6.6).
Thus, in penicillin fermentation contamination is a main constraint. Hence, one has to be careful in preventing contamination. This was the one of the main problems during early times of penicillin production, when fermentation was carried out in bottles and contamination in one bottle may destroy penicillin in entire batch of bottles.
In the typical penicillin fermentation there is a growth of 10 hrs duration with a doubling time of 6 hrs during which the greater part of the cell mass is formed. The oxygen supply in the growing culture is critical since the increasing viscosity hinders oxygen transfer. After growth phase, the culture proceeds to actual penicillin production. The growth is sharply reduced by feeding with various culture medium components.
The production phase can be extended to 120- 180 hrs. Penicillin production by continuous fermentation has been attempted but it has been difficult due to instability of the production strains. A batch fill and draw system has been suggested as an alternative. In this process 20-40% of the fermentation contents is drawn off and replaced with fresh nutrient solution. This process may be repeated up to 10 without affecting yield.
4. Extraction and Purification:
After it is assessed that sufficient amount of penicillin has been produced during fermentation process, it is extracted and then purified.
The entire process is carried out in three different stages.
They are:
(a) Separation of mycelium
(b) Extraction of penicillin and
(c) Treatment of crude extract
(a) Separation of Mycelium:
Mycelium is separated from the medium by employing rotatory vacuum filter. This process should be performed carefully in order to avoid contaminating microorganisms which produce penicillinase enzyme, degrading the penicillin.
(b) Extraction of Penicillin:
The penicillin is excreted into the medium and less than 1% remains as mycelium bound. Extraction of penicillin is carried out by employing counter current extraction method. The pH of the liquid after separation of the mycelium is adjusted to 2.0 to 2.5 by adding phosphoric or sulphuric acid. This treatment converts penicillin into anionic form.
The liquid is immediately extracted with an organic solvent such as amylacetate or butylacetate or methyl isobutyl ketone. This step has to be carried out quickly because penicillin is quite unstable at low pH values. Podbielniak counter current extractor is used for this purpose. The penicillin is then back extracted into water from the organic solvent by adding enough potassium or sodium hydroxide which also results in the elevation of pH to 7.0 to 7.5.
The resulting aqueous solution is again acidified and re-extracted with organic solvent. These shifts between the water and the solvent help in the purification of the penicillin. Finally, the penicillin is obtained in the form of sodium penicillin. The spent solvent is recovered by distillation for reuse.
(c) Treatment of Crude Extract:
The resulted sodium penicillin is treated with charcoal to remove pyrogens (fever causing substances). It is also, sometimes, sterilized to remove bacteria by using Seitz filter. Then, the sodium penicillin is prepared in crystalline form by crystallization. It may be packed as powder in sterile vials or prepared in the form of tablets or in the form of syrups for oral usage. The pharmaceutical grade may be used in the production of semi synthetic penicillin.
Uses of Penicillin:
1. Most of the penicillin’s are active against Gram-positive bacteria, in which they inhibit the cell wall synthesis leading to the death of bacteria.
2. Used therapeutically in the treatment of infectious diseases of humans caused by Gram (+) positive bacteria.