In this article we will discuss about Animal Cell Culture:- 1. History of Animal Cell Culture 2. Requirements for Animal Cell Culture 3. Types 4. Characteristics 5. Advantages 6. Disadvantages 7. Applications.
Contents:
- History of Animal Cell Culture
- Requirements of Animal Cell Culture
- Types of Animal Cell Culture
- Characteristics of Cultured Animal Cells
- Advantages of Animal Cell Culture
- Disadvantages of Animal Cell Culture
- Applications of Animal Cell Culture
1. History of Animal Cell Culture:
Although animal cell culture was first successfully undertaken by Ross Harrison in 1907, it was not until the late 1940’s to early 1950’s that several developments occurred that made cell culture widely available as a tool for scientists.
First, there was the development of antibiotics that made it easier to avoid many of the contamination problems that plagued earlier cell culture attempts.
Second was the development of the techniques, such as the use of trypsin to remove cells from culture vessels, necessary to obtain continuously growing cell lines (such as HeLa cells).
Third, using these cell lines, scientists were able to develop standardized, chemically defined culture media that made it far easier to grow cells.
Historical Events in the Development of Cell Culture:
1878:
Claude Bernard proposed that physiological systems of an organism can be maintained in a living system after the death of an organism.
1885:
Roux maintained embryonic chick cells in a saline culture.
1897:
Loeb demonstrated the survival of cells isolated from blood and connective tissue in serum and plasma.
1903:
Jolly observed cell division of salamander leucocytes in vitro.
1907:
Harrison cultivated frog nerve cells in a lymph clot held by the ‘hanging drop’ method and observed the growth of nerve fibres in vitro for several weeks. He was considered by some as the father of cell culture.
1910:
Burrows succeeded in long-term cultivation of chicken embryo cell in plasma clots. He made detailed observation of mitosis.
1911:
Lewis and Lewis made the first liquid media consisted of sea water, serum, embryo extract, salts and peptones. They observed limited monolayer growth.
1913:
Carrel introduced strict aseptic techniques so that cells could be cultured for long periods.
1916:
Rous and Jones introduced proteolytic enzyme trypsin for the subculture of adherent cells.
1923:
Carrel and Baker developed ‘Carrel’ or T-flask as the first specifically designed cell culture vessel. They employed microscopic evaluation of cells in culture.
1927:
Carrel and Rivera produced the first viral vaccine – Vaccinia.
1933:
Gey developed the roller tube technique.
1940s:
The use of the antibiotics penicillin and streptomycin in culture medium decreased the problem of contamination in cell culture.
1948:
Earle isolated mouse L fibroblasts which formed clones from single cells. Fischer developed a chemically defined medium, CMRL 1066.
1949:
Enders reported that polio virus could be grown on human embryonic cells in culture.
1952:
Gey established a continuous cell line from a human cervical carcinoma known as HeLa (Helen Lane) cells. Dulbecco developed plaque assay for animal viruses using confluent monolayers of cultured cells.
1954:
Abercrombie observed contract inhibition: motility of diploid cells in monolayer culture ceases when contact is made with adjacent cells.
1955:
Eagle studied the nutrient requirements of selected cells in culture and established the first widely used chemically defined medium.
1961:
Hay flick and Moorhead isolated human fibroblasts (WI-38) and showed that they have a finite life-span in culture.
1964:
Littlefield introduced the HAT medium for cell selection.
1965:
Ham introduced the first serum-free medium which was able to support the growth of some cells.
1965:
Harris and Watkins were able to fuse human and mouse cells by the use of a virus.
1975:
Kohler and Milstein produced the first hybridoma capable of secreting a monoclonal antibody.
1978:
Sato established the basis for the development of serum-free media from cocktails of hormones and growth factors.
1982:
Human insulin became the first recombinant protein to be licensed as a therapeutic agent.
1985:
Human growth hormones produced from recombinant bacteria was accepted for therapeutic use.
1986:
Lymphoblastoidy γlFN licensed.
1987:
Tissue-type plasminogen activator (tPA) from recombinant animal cells became commercially available.
1989:
Recombinant erythropoietin in trial.
1990:
Recombinant products in clinical trial (HBsAG, factor VIII, HIVgp120, CD4, GM-CSF, EGF, mAbs, IL-2).
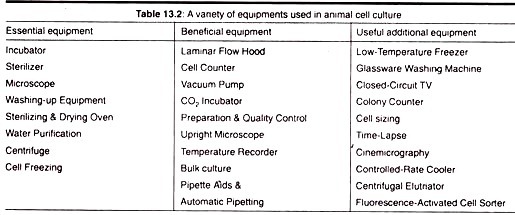
2. Requirements for Animal Cell Culture:
a. Sterile Work Area:
Where possible, a separate room should be made available for clean cell culture work. This room should be free of “through traffic” and, if possible, equipped with an air flow cabinet which supplies filtered air around the work surface.
A HEPA (High Efficiency Particle Air Filter) filtered air supply is desirable but not always affordable. Primary animal tissue and micro-organisms must not be cultured in or near the cell culture laboratory and the laboratory must be specifically designated for clean cell culture work.
b. Incubation Facilities:
In addition to an airflow cabinet and benching which can be easily cleaned, the cell culture laboratory will need to be furnished with an incubator or hot room to maintain the cells at 30-40°C. The incubation temperature will depend on the type of cells being cultivated. Insect cells will grow best at around 30°C while mammalian cells require a temperature of 37°C.
c. Refrigerators and Freezer:
Both items are very important for storage of liquid media at 4°C and for enzymes (e.g., trypsin) and some media components (e.g., glutamine and serum) at -20°C. A refrigerator or cold room is required to store medium and buffers.
A freezer will be needed for keeping pre aliquot stocks of serum, nutrients and antibiotics. Reagents may be stored at a temperature of -20°C but if cells are to be preserved it may be necessary to provide liquid nitrogen or a -70°C freezer.
d. Microscopes:
A simple inverted microscope is essential so that cultures can be examined in flasks and dishes. It is vital to be able to recognize morphological changes in cultures since these may be the first indication of deterioration of a culture.
A very simple light microscope with x 100 magnification will suffice for routine cell counts in a haemocytometer, although a microscope of much better quality will be required for chromosome analysis or autoradiography work.
e. Tissue Culture Ware:
A variety of tissue culture plastic-ware is available, the most common being specially treated polystyrene. Although all tissue culture plastic-ware should support cell growth adequately, it is essential when using a new supplier or type of dish to ensure that cultures grow happily in it.
The tests to ensure this, such as growth curves and time of reaching a confluent monolayer, are similar, to those used to ensure that serum batches are satisfactory. Cells can be maintained in Petri dishes or flasks (25 cm2 or 75 cm2) which have the added advantage that the flasks can be gassed and then sealed so that a CO2 incubator need not be used. This is particularly useful if incubators fail. Tissue culture ware is always chosen to match the procedure.
f. Washing Up and Sterilizing Facilities:
Availability of a wide range of plastic tissue culture reduces the amount of necessary washing up. However, glassware such as pipettes should be soaked in a suitable detergent, then passed through a stringent washing procedure with thorough soaking in distilled water prior to drying and sterilizing.
Pipettes are often plugged with non-absorbent cotton wool before putting into containers for sterilizing. Glassware, such as pipettes, conical flasks, beakers (covered with aluminum foil) are sterilized in a hot air oven at 160°C for one hour.
All other equipment, such as automatic pipette tips and bottles (lids loosely attached) are autoclaved at 121°C for 20 min. Sterilizing indicators such as sterile test strip are necessary for each sterilizing batch to ensure that the machine is operating effectively. Autoclave bags are available for loose items. Aluminum foil also makes good packaging material.
g. Liquid Nitrogen Deep Freezer:
Invariably for continuous and finite cell lines, samples of cultures will need to be frozen down for storage. It is important to maintain continuity in cells to prevent genetic drift and to guard against loss of the cell line through contamination and other disasters.
The procedure for freezing cells is general for all cells in culture. They should be frozen in exponential phase of growth with a suitable preservative, usually dimethylsulfoxide (DMSO). The cells are frozen slowly at 1°C/min to -50°C and then kept either at -196°C immersed in liquid N2 (in sealed glass ampoules) or above the liquid surface in the gas phase (screw top ampoules). Deterioration of frozen cells has been observed at -70°C, therefore, -196°C (liquid N2) seems to be necessary.
To achieve slow freezing rates a programmable freezer or an adjustable neck plug or freezing tray for use in a narrow-necked liquid nitrogen freezer can be used. Alternatively, ampoules may be frozen in a polystyrene box with 1″ thick walls. This will insulate the ampoules to slow the freezing process to 1°C/min in a -70°C freezer.
h. Water Still or Reverse Osmosis Apparatus:
A double distilled or reverse osmosis water supply is essential for preparation of media, and rinsing glassware. The pH of the double distilled water should be regularly checked as in some cases this can vary. Variations in the quality of water used may account for variation in results.
Therefore, water from one source should be used. Water is sterilized by autoclaving at 121°C for 20 min. The distilled water must be glass distilled and stored in glass if it is to be used for the preparation of media. Storage in plastic may result in leaching of toxic substances from the plastic into the water.
i. Filter Sterilization:
Media that cannot be autoclaved must be sterilized through a 0.22 mm pore size membrane filter. These are obtainable in various designs to allow a wide range of volumes to be filtered (e.g., Millipore, Gelman).
They can be purchased as sterile, disposable filters, or they may be sterilized by autoclaving in suitable filter holders. Culture media, enzymes, hormones, cofactors and bicarbonate buffers are examples of non-autoclavable substances.
j. Other Requirements:
(a) Temperature:
In most of the mammalian cell cultures, the temperature is maintained at 37°C in the incubators as the body temperature of Homo sapiens is 37°C.
(b) Culture Media:
The culture media is prepared in such a way that it provides:
a. The optimum conditions of factors like pH, osmotic pressure, etc.
b. It should contain chemical constituents which the cells or tissues are incapable of synthesizing.
Generally the media is the mixture of in- Organic salts and other nutrients capable of sustaining cells in culture such as amino acids, fatty acids, sugars, ions, trace elements,vitamins, cofactors, and ions. Glucose is added as energy source—its concentration varies depending on the requirement.
Phenol Red is added as a pH indicator of the medium. There are two types of media used for culture of animal cells and tissues:
(a) Natural Media:
The natural media are the natural sources of nutrient sufficient for growth and proliferation of animal cells and tissues.
The Natural Media used to Promote Cell Growth Fall in Three Categories:
1. Coagulant, such as plasma clots. It is now commercially available in the form of liquid plasma kept in silicon ampoules or lyophilized plasma. Plasma can also be prepared in the laboratory taking out blood from male fowl and adding heparin to prevent blood coagulation.
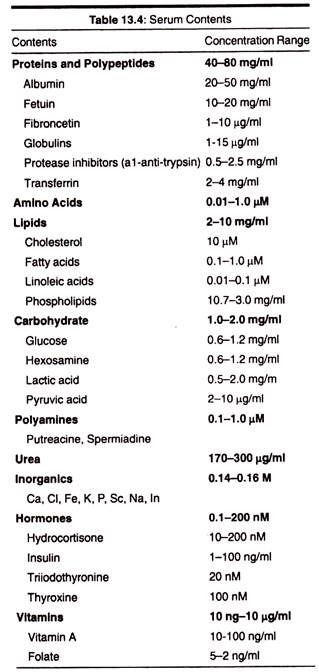
2. Biological fluids such as serum. Serum is one of the very important components of animal cell culture which is the source of various amino acids, hormones, lipids, vitamins, poly- amines, and salts containing ions such as calcium, ferrous, ferric, potassium, etc.
It also contains the growth factors which promotes cell proliferation, cell attachment and adhesion factors. Serum is obtained from human adult blood, placental, cord blood, horse blood, calf blood. The other forms of biological fluids used are coconut water, amniotic fluid, pleural fluid, insect haemolymph serum, culture filtrate, aqueous humour, from eyes etc.
3. Tissue extracts for example Embryo extracts- Extracts from tissues such as embryo, liver, spleen, leukocytes, tumour, bone marrow etc. are also used for culture of animal cells.
(b) Synthetic Media:
Synthetic media are prepared artificially by adding several organic and inorganic nutrients, vitamins, salts, serum proteins, carbohydrates, co-factors, etc. Different types of synthetic media can be prepared for a variety of cells and tissues to be cultured.
Synthetic Media are of Two Types:
1. Serum containing media (media containing serum)
2. Serum- free media (media without serum).
Examples of some media are: minimal essential medium (MEM), RPMI 1640 medium, CMRL 1066, F12, etc.
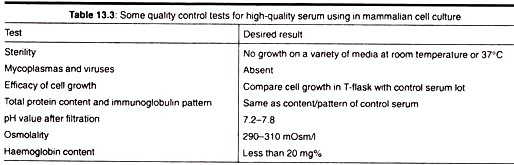
Advantages of Serum in Culture Medium are:
1. Serum binds and neutralizes toxins,
2. Serum contains a complete set of essential growth factors, hormones, attachment and spreading factors, binding and transport proteins, Contents
3. It contains the protease inhibitors,
4. It increases the buffering capacity,
5. It provides trace elements.
Disadvantages of Serum in Culture Medium are:
a. It is not chemically defined, and therefore, its composition varies a lot,
b. It is sometimes source of contamination by viruses, mycoplasma, prions, etc.,
c. It increases the difficulties and cost of downstream processing,
d. It is the most expensive component of the culture medium.
(c) pH:
Most media maintain the pH between 7 and 7.4. A pH below 6.8 inhibits cell growth. The optimum pH is essential to maintain the proper ion balance, optimal functioning of cellular enzymes and binding of hormones and growth factors to cell surface receptors in the cell cultures. The regulation of pH is done using a variety of buffering systems. Most media use a bicarbonate CO2 system as its major component.
(d) Osmolality:
A change in osmolality can affect cell growth and function. Salt, Glucose and Amino acids in the growth media determine the osmolality of the medium. All commercial media are formulated in such a way that their final osmolality is around 300 mOsm.
(e) Buffering:
Carbon dioxide in the medium is in a developed state and the concentration depends on the atmospheric carbon dioxide tension and temperature. The CO2 exists in the medium in the form of carbonic acid and protons.
CO2 + H2O → H2CO3← H+ + HCO3−
It must be noted that there is a direct relationship between the concentration of carbon dioxide, bicarbonate ions and pH of the media. By increasing the atmospheric CO2, the pH will be reduced making the medium acidic.
(f) Growth Factors:
In addition to buffering the medium, there are other growth requirements including amino acids, the requirement for which may vary with cell culture type. Commonly the necessary amino acids include cysteine and tyrosine, but some non-essential amino acids may be needed.
Glutamine is also required by most cell lines and it has been suggested that cultured cells use glutamine as an energy and carbon source in preference to glucose, although glucose is present in most defined media. Glutamine is usually added at a final concentration of 2 mM, however, once added to the medium the glutamine is only stable for about 3 weeks at 4°C.
(g) Antibiotics and Antimycotics:
Unless good sterile conditions can be maintained (e.g., using laminar flow hoods) it is necessary to incorporate antibiotics and antimycotics into the media. A wide range of suitable preparations are available from relatively specific antibiotics, e.g., penicillin/streptomycin solutions, to broader spectrum antibacterial/antimycotic agents such as kanamycin or amphotericin B.
The antibiotics chosen should clearly not to be toxic to the cells in culture and may depend on the type of contamination experienced in the individual laboratory.
3. Types of Animal Cell Culture:
i. Primary Culture:
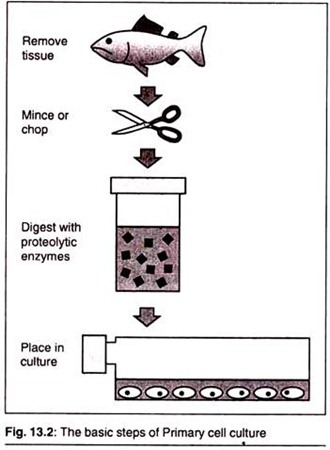
When cells are surgically removed from an organism and placed into a suitable culture environment, they will attach, divide and grow. This is called a Primary Culture. There are two basic methods for doing this.
First, for Explant Cultures, small pieces of tissue are attached to a glass or treated in a plastic culture vessel and bathed in culture medium. After a few days, individual cells will move from the tissue explant onto the culture vessel surface or substrate where they will begin to divide and grow.
The second, more widely used method, speeds up this process by adding digesting (proteolytic) enzymes, such as trypsin or collagenase, to the tissue fragments to dissolve the cement holding the cells together. This creates a suspension of single cells that are then placed into culture vessels containing culture medium and allowed to grow and divide. This method is called Enzymatic Dissociation.
Advantages of Primary Cell Culture:
The major advantages of primary cultures are the retention of:
1. The Capacity for Biotransformation:
In many cases, the metabolism of a primary cell culture has greater similarity to in vivo than that seen with sub-cellular fractions used as an exogenous source for biotransformation.
2. The Tissue-Specific Functions:
The second advantage of primary cultures is the retention of tissue specific functions. For example, primary cultures of rat myocardial cells consisting of synchronously beating cells can be prepared. When these cultures were exposed to tricyclic antidepressants that are cardio toxic, beating were observed.
Limitations of Primary Cell Cultures:
One limitation of primary cultures is the necessity to isolate cells for each experiment. Procedures to isolate cells require the disruption of the tissue, often with proteolytic enzymes. This may result in the loss or damage of specific membrane receptors, damage to the integrity of the membrane, and loss of cellular products.
ii. Sub-Culturing:
When the cells in the primary culture vessel have grown and filled up all of the available culture substrate, they must be Sub-cultured to give them room for continued growth. This is usually done by removing them as gently as possible from the substrate with enzymes. These are similar to the enzymes used in obtaining the primary culture and are used to break the protein bonds attaching the cells to the substrate.
Some cell lines can be harvested by gently scraping the cells off the bottom of the culture vessel. Once released, the cell suspension can then be subdivided and placed into new culture vessels.
Once a surplus of cells is available, they can be treated with suitable cryoprotective agents, such as dimethylsulfoxide (DMSO) or glycerol, carefully frozen and then stored at cryogenic temperatures (below -130°C) until they are needed. The theory and techniques for cryopreserving cells are covered in the Corning Technical Bulletin: General Guide for Cryogenically Storing Animal Cell Cultures.
4. Characteristics of Cultured Animal Cells:
i. Cell Culture Systems:
Two basic culture systems are used for growing cells. These are based primarily upon the ability of the cells to either grow attached to a glass or treated plastic substrate, called as monolayer culture systems, or floating free in the culture medium called as Suspension Culture Systems.
Monolayer cultures are usually grown in tissue culture treated dishes, T-flasks, roller bottles, Cell-STACK® Culture Chambers, or multiple well plates, the choice being based on the number of cells needed, the nature of the culture environment, cost and personal preference.
Suspension Cultures are usually Grown Either:
1. In magnetically rotated spinner flasks or shaken Erlenmeyer flasks where the cells are kept actively suspended in the medium;
2. In stationary culture vessels such as T-flasks and bottles where, although the cells are not kept agitated, they are unable to attach firmly to the substrate. Many cell lines, especially those derived from normal tissues, are considered to be Anchorage-Dependent, that is, they can only grow when attached to a suitable substrate.
Some cell lines that are no longer considered normal (frequently designated as Transformed Cells) are frequently able to grow either attached to a substrate or floating free in suspension; they are Anchorage-Independent. In addition, some normal cells, such as those found in the blood, do not normally attach to substrates and always grow in suspension.
ii. Types of Cells:
Cultured cells are usually described based on their morphology (shape and appearance) or their functional characteristics.
There are three basic morphologies:
1. Epithelial:
Like: cells that are attached to a substrate and appear flattened and polygonal in shape.
2. Lymphoblast-Like:
Cells that do not attach normally to a substrate but remain in suspension with a spherical shape.
3. Fibroblast-Like:
Cells that are attached to a substrate and appear elongated and bipolar, frequently forming swirls in heavy cultures. It is important to remember that the culture conditions play an important role in determining shape and that many cell cultures are capable of exhibiting multiple morphologies.
Using cell fusion techniques, it is also possible to obtain hybrid cells by fusing cells from two different parents. These may exhibit characteristics of either parent or both parents. This technique was used in 1975 to create cells capable of producing custom tailored monoclonal antibodies.
These hybrid cells (called Hybridomas) are formed by fusing two different but related cells. The first is a spleen-derived lymphocyte that is capable of producing the desired antibody. The second is a rapidly dividing myeloma cell (a type of cancer cell) that has the machinery for making antibodies but is not programmed to produce any antibody.
The resulting hybridomas can produce large quantities of the desired antibody. These antibodies, called Monoclonal Antibodies due to their purity, have many important clinical, diagnostic, and industrial applications with a yearly value of well over a billion dollars.
iii. Functional Characteristics:
The characteristics of cultured cells result from both their origin (liver, heart, etc.) and how well they adapt to the culture conditions. Biochemical markers can be used to determine if cells are still carrying on specialized functions that they performed in vivo (e.g., liver cells secreting albumin).
Morphological or ultra-structural markers can also be examined (e.g., beating heart cells). Frequently, these characteristics are either lost or changed as a result of being placed in an artificial environment. Some cell lines will eventually stop dividing and show signs of aging.
These lines are called Finite. Other lines which become immortal can continue to divide indefinitely and are called Continuous cell lines. When a “normal” finite cell line becomes immortal, it undergoes a fundamental irreversible change or “transformation”. This can occur spontaneously or be brought about intentionally using drugs, radiation or viruses.
Transformed Cells are usually easier and faster growing, may often have extra or abnormal chromosomes and frequently can be grown in suspension.Cells that have the normal number of chromosomes are called Diploid cells; those that have other than the normal number are Aneuploid. If the cells form tumours when they are injected into animals, they are considered to be Neo-plastically Transformed.
5. Advantages of Animal Cell Culture:
a. Controlled physiochemical environment (pH, temperature, osmotic pressure, O2, etc.)
b. Controlled and defined physiological conditions
c. Homogeneity of cell types (achieved through serial passages)
d. Economical, since smaller quantities of reagents are needed than in vivo.
e. Legal, moral and ethical questions of animal experimentation are avoided.
6. Disadvantages of Animal Cell Culture:
a. Expertise is needed, so that behaviour of cells in culture can be interpreted and regulated.
b. Ten times more expensive for same quantity of animal tissue; therefore, reasons for its use should be compelling.
c. Unstable aneuploid chromosome constitution.
7. Applications of Animal Cell Culture:
A. Model Systems:
Cell cultures provide a good model system for studying;
a. Basic cell biology and biochemistry.
b. The interactions between disease-causing agents and cells.
c. The effects of drugs on cells.
d. The process and triggers for aging.
f. Nutritional studies.
B. Toxicity Testing:
Cultured cells are widely used alone or in conjunction with animal tests to study the effects of new drugs, cosmetics and chemicals on survival and growth in a wide variety of cell types. Especially important are liver and kidney derived cell cultures.
C. Cancer Research:
Since both normal cells and cancer cells can be grown in culture, the basic differences between them can be closely studied. In addition, it is possible, by the use of chemicals, viruses and radiation, to convert normal cultured cells to cancer causing cells.
Thus, the mechanisms that cause the change can be studied. Cultured cancer cells also serve as a test system to determine suitable drugs and methods for selectively destroying types of cancer.
D. Virology:
One of the earliest and major uses of cell culture is the replication of viruses in cell cultures (in place of animals) for use in vaccine production. Cell cultures are also widely used in the clinical detection and isolation of viruses, as well as basic research into how they grow and infect organisms.
E. Cell-Based Manufacturing:
While cultured cells can be used to produce many important products, three areas are generating the most interest.
The first is the large-scale production of viruses for use in vaccine production. These include vaccines for polio, rabies, chicken pox, hepatitis B and measles.
The second is the large-scale production of cells that have been genetically engineered to produce proteins that have medicinal or commercial value. These include monoclonal antibodies, insulin, hormones, etc. The third is the use of cells as replacement tissues and organs. Artificial skin for use in treating burns and ulcers is the first commercially available product.
However, testing is underway on artificial organs such as pancreas, liver and kidney. A potential supply of replacement cells and tissues may come out of work currently being done with both embryonic and adult stem cells.
These are cells that have the potential to differentiate into a variety of different cell types. It is hoped that learning how to control the development of these cells may offer new treatment approaches for a wide variety of medical conditions.
F. Genetic Counselling:
Amniocentesis, a diagnostic technique that enables doctors to remove and culture fetal cells from pregnant women, has given doctors an important tool for the early diagnosis of fetal disorders. These cells can then be examined for abnormalities in their chromosomes and genes using karyotyping, chromosome painting and other molecular techniques.
G. Genetic Engineering:
The ability to transfect or reprogram cultured cells with new genetic material (DNA and genes) has provided a major tool to molecular biologists wishing to study the cellular effects of the expression of these genes (new proteins).
These techniques can also be used to produce these new proteins in large quantity in cultured cells for further study. Insect cells are widely used as miniature cells factories to express substantial quantities of proteins that they manufacture after being infected with genetically engineered baculoviruses.
H. Drug Screening and Development:
Cell-based assays have become increasingly important for the pharmaceutical industry, not just for cytotoxicity testing but also for high throughput screening of compounds that may have potential use as drugs. Originally, these cell culture tests were done in 96 well plates, but increasing use is now being made of 384 and 1536 well plates.
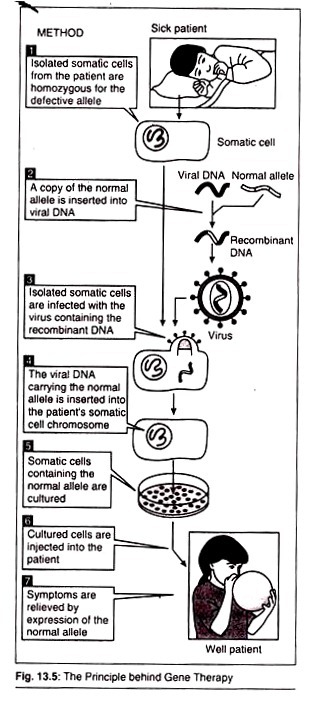
I. Gene Therapy:
In modern molecular biology, Gene Therapy is an experimental technique that involves insertion of cloned/altered genes into cells using r-DNA technology to replace defective genes causing genetic abnormalities or to prevent potential disorders.
Followings are the purpose of Gene Therapy:
a. Swapping harmful mutant alleles with functional ones by selective reverse mutation.
b. Deactivating improperly functioning mutated gene.
c. Inserting a new gene into the body to help battle a disease.
d. Interchanging non-functional gene with normal gene through homologous recombination.